You are here: Home: LCU 3 | 2005 : Howard West, MD
Howard West, MD |
EDITED COMMENTS |
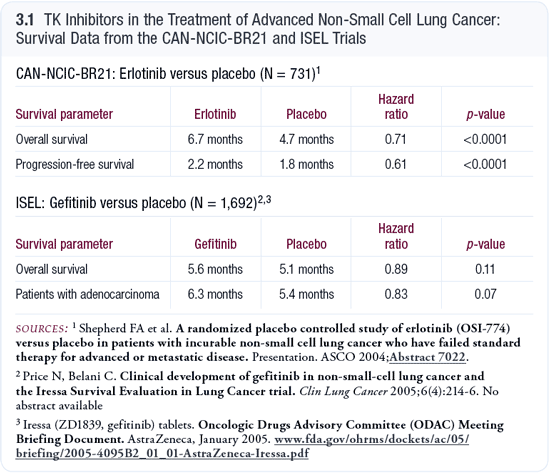
 CAN-NCIC-BR21: Randomized trial of erlotinib versus observation in patients with previously treated advanced NSCLC CAN-NCIC-BR21: Randomized trial of erlotinib versus observation in patients with previously treated advanced NSCLC
CAN-NCIC-BR21 (3.1), with over 600 patients, evaluated subsets of patients and demonstrated an interesting dissociation between response rates, which were clearly and consistently higher in women, nonsmokers, Asians and patients with adenocarcinoma, and survival benefit associated with erlotinib 150 mg, which seemed to extend over a much broader range. The survival curves shifted the same, regardless of whether the patients were male or female and had squamous or adenocarcinoma. The hazard ratios and relative benefits were essentially identical.
It’s also interesting that a response was not necessary for a survival benefit. This was suggested from the second-line trials of docetaxel, which demonstrated a single-digit response rate but a better one-year survival compared to best supportive care (Shepherd 2000). Also, pemetrexed, when compared to docetaxel, has superimposable activities and, presumably, the same clinical benefit with an almost negligible response rate (Hanna 2004). Perhaps the bar is too high, at least in advanced lung cancer, to expect that a 50 percent reduction in tumor size is necessary to translate into a survival benefit. Prolonged stable disease also helps.
ISEL: Randomized trial of gefitinib versus observation in patients with previously treated advanced NSCLC
I think everyone in the field was stunned that the ISEL trial of gefitinib 250 mg, which had a similar design to CAN-NCI-BR21, was negative. Nearly 1,700 patients were enrolled (Price 2005; [3.1]). Everyone suspected that the two drugs — erlotinib and gefitinib — were close enough to interchange and that the ISEL trial would have a positive result. ISEL was a large trial, and it had a very good trial design. It was surprising to us that it was negative, with only trends in the right direction.
Rash and antitumor effect of EGFR inhibitors
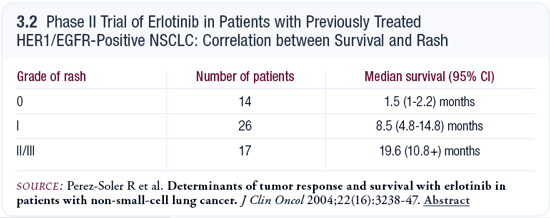
The association between rash and survival has been noted with erlotinib for years and across a wide range of tumor types. Roman Perez-Soler demonstrated a striking difference in median survival — 1.5 months for patients who had no rash versus 19.6 months for patients who had a Grade II or III rash. The median survival for patients with a Grade I rash fell right in between (Perez-Soler 2004; [3.2]). Many trials have shown a similar “rash-dependent” stratification with survival.
It is less clear with gefitinib, and I think that has contributed to the concept of using lower doses of gefitinib. In SWOG-S0126, the gefitinib trial in patients with BAC that I led, we found that the development of rash was significantly associated with better survival. There was some stepwise association with a higher degree of rash, but it was not as clear (West 2004). I have been struck by the consistency of the data in a wide range of trials with EGFR inhibitors, especially erlotinib. Even in other settings, trials of cetuximab have shown similar trends (Saltz 2003).
In terms of the relationship between rash and response rates, some trials have evaluated it and others have not. In both SWOG-S0126 (West 2004) and a trial of erlotinib in 78 evaluable patients with BAC presented by Mark Kris at ASCO (Kris 2004), we saw no responses among the patients who failed to develop a rash. A fundamental question is whether you can obtain a better response by increasing the dose of the drug and dosing to rash. My take is that it seems to be more indigenous to the patient. If you consider the Phase I gefitinib trials, not everyone developed a rash by increasing the dose to 800 or 1,000 mg.
Management of patients with BAC
We held a consensus conference about BAC in New York in November 2004. Our consensus was that there were no data to define the value of chemotherapy. Anecdotally, in clinical experience, chemotherapy is viewed as less effective for patients with BAC than other forms of lung cancer. These days, chemotherapy is often skipped in favor of EGFR-TK inhibitors.
I think that is an acceptable potential standard of care. However, in patients with a good performance status, I still treat them first with standard chemotherapy and move on immediately to erlotinib. In a patient with a more marginal performance status, I might use an EGFR-TK inhibitor immediately.
Integration of erlotinib into the management of patients with Stage IV NSCLC
Certainly, for patients who either have clinical or molecular evidence of carrying the EGFR gene mutation, you might make a strong argument to use erlotinib as first-line therapy. The TRIBUTE trial, which evaluated carboplatin/paclitaxel with or without erlotinib as first-line therapy, demonstrated a survival benefit for nonsmokers who received erlotinib (Miller 2004). I believe some institutions, including Memorial Sloan-Kettering, are using chemotherapy plus concurrent erlotinib for nonsmokers.
Although a survival benefit was seen in the nonsmokers in the TRIBUTE trial, I don’t think that answers the question of how well these patients would have done with a sequential, instead of concurrent, approach. I think the data suggesting an antagonistic interaction between conventional chemotherapy and the EGFR-TK inhibitors (ie, the INTACT [Herbst 2004a, Giaccone 2004], TALENT [Gatzemeier 2004] and TRIBUTE [Herbst 2004b] trials) would dissuade me from using concurrent chemotherapy and an EGFR-TK inhibitor as first-line therapy.
Obviously, a much larger population of patients will be receiving erlotinib in the second- or third-line setting or beyond. In that situation, we have other approved agents — docetaxel and pemetrexed. For patients with a performance status that allows more chemotherapy, I tend to use chemotherapy in the second-line setting and sometimes in the third-line setting because you don’t need a huge physiologic reserve to tolerate the EGFR-TK inhibitors. Some patients may be able to receive chemotherapy second line and erlotinib as third-line therapy, but they may not be able to do the reverse.
I would generally choose between chemotherapy and an EGFR-TK inhibitor as salvage therapy, based on factors like performance status and smoking status. I usually give nonsmokers an EGFR-TK inhibitor early on to determine if they would have a prolonged benefit with minimal toxicity. Another important potential factor is the patient’s prior response to chemotherapy.
In patients who have had a response or prolonged stable disease and a good performance status, I would be more inclined to use chemotherapy before an EGFR-TK inhibitor even in the salvage setting. In patients who have had rapid progression on chemotherapy, I might be inclined to try a different approach and switch over to an EGFR-TK inhibitor earlier.
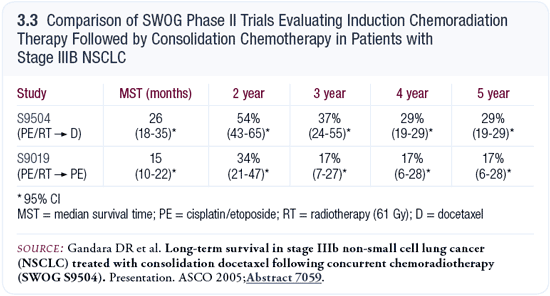
SWOG-S9504: Consolidation docetaxel after concurrent chemoradiation therapy in patients with Stage III disease
I use the SWOG-S9504 docetaxel consolidation approach in patients with Stage III disease. Although S9504 was a Phase II trial, there aren’t enough Phase III trials with contemporary approaches in patients with Stage III lung cancer to guide us. By necessity, we need to extrapolate from the available data. To me, the data from SWOG-S9504 have been strikingly superior to those preceding that trial (Gandara 2003; [3.3]).

Continuation of bevacizumab on disease progression
If bevacizumab becomes integrated into first-line therapy, it will raise the ongoing question that we have in a huge range of oncology practice: How long should targeted therapies be continued, even in patients who progress on a combination of targeted therapy plus conventional chemotherapy?
There has been one prominent Phase II trial of bevacizumab and erlotinib that looked encouraging (Herbst 2005), and further study with that combination is ongoing. That combination may be employed as a salvage therapy in patients who have already been on bevacizumab in the first-line setting. The Southwest Oncology Group is planning to undertake a trial of pemetrexed with bevacizumab in the salvage setting, so that will provide important clinical activity and toxicity data for that combination. I’m sure docetaxel and bevacizumab will also be studied extensively (3.4).
It would be nice to actually see some suggestion of a survival benefit beyond that seen with chemotherapy or erlotinib alone. Another key component will be showing that there isn’t prohibitive toxicity from these combinations. I would be very cautious about combining bevacizumab with anything that hasn’t been extensively tested.
Evolution of clinical trial data with adjuvant chemotherapy
At ASCO 2003 (Le Chevalier 2003), the IALT trial data were presented at a plenary session with statistically and arguably clinically significant results. A four percent overall survival benefit was enough to convince the people who already believed in the concept of systemic therapy for early-stage lung cancer. The skeptics remained largely unconvinced, and we still debated questions about treating patients with Stage IB disease. These patients seemed to derive a little less benefit in the IALT trial than patients with Stage II or III disease. It was still very much an open question: Which patients should be recommended for adjuvant chemotherapy? Much of that debate was laid to rest at ASCO in 2004, when two trials — presented back to back in oral sessions — showed double-digit survival benefits (Strauss 2004; Winton 2004). Everyone would have to agree that these data were quite clinically significant. One trial was with a chemotherapy regimen that was widely used in the United States — carboplatin and paclitaxel — and that same trial specifically evaluated patients with Stage IB disease.
We believed it would be difficult to demonstrate a survival benefit in patients with Stage IB disease in the adjuvant setting; however, a double-digit survival benefit of 12 percent was demonstrated at four years. So at this point, I believe adjuvant chemotherapy has become the clear standard of practice and just about every patient should at least have systemic therapy discussed, if not implemented. For some patients, getting through a thoracotomy alone is a challenge. Those patients who were enrolled on the trial had already been selected as potential candidates. So it is not necessarily for everybody, but with the magnitude of the benefits it deserves to be discussed. I would find fault with the rare surgeon or practitioner who dissuades their patient after surgery from at least considering a consultation with a medical oncologist to consider adjuvant chemotherapy.
Selection of adjuvant chemotherapeutic regimens
The potential curability of the disease makes me try to adhere as much as possible to regimens with maximum efficacy. The clinical trials have used a range of cisplatin-based chemotherapy in addition to carboplatin and paclitaxel.
The few trials that have directly compared cisplatin- and carboplatin-based regimens in other settings in lung cancer have suggested a slight efficacy advantage for cisplatin-based chemotherapy. I favor cisplatin-based regimens if a patient’s performance status would allow it, which is really a subset of patients. Although it may seem anachronistic, I use as much cisplatin and vinorelbine as any other regimen in this setting, based on it being one of the leading regimens in the IALT trial and the regimen that was employed in the NCI Canada JBR.10 trial.
I would have no trouble using almost any platinum-based doublet, and probably in around 50 percent of my patients, carboplatin-based doublets are a much more feasible choice. I would have no reluctance in utilizing carboplatin and paclitaxel now that there are data supporting its comparability in this setting. If feasible, I’d prefer to use the regimens that have supporting data. However, given the large volume of data in advanced disease that show essentially complete comparability of these platinum doublets, most physicians in the field would consider them to be interchangeable.
Select publications
 |
Dr West is Director of Medical Therapeutics in Thoracic Oncology at the Swedish Cancer Institute in Seattle, Washington. |
|

