|
You are here: Home: LCU 3 | 2005 : Rogerio C Lilenbaum, MD
Rogerio C Lilenbaum, MD |
EDITED COMMENTS |
 ECOG trial E4599: Carboplatin/paclitaxel with or without bevacizumab ECOG trial E4599: Carboplatin/paclitaxel with or without bevacizumab
Until now, every time we talked about clinical research in advanced non-small cell lung cancer, we would have to say, “We’ve been on a plateau for at least the last five years, if not longer, with platinum doublets and benchmark median one-year survival rates, etcetera, with a small percentage of people who would live two years from the time of diagnosis.”
The bevacizumab trial breaks through that plateau for the very first time (Sandler 2005). Our group in Miami entered over 30 patients in the ECOG-E4599 trial, and we had the largest accrual in CTSU. So we had a lot of exposure to that regimen. What the study shows is not unlike the data we saw in colon and breast cancer. I’m not sure exactly how, but something about bevacizumab enhances the activity of chemotherapy regimens. We all know about the anti-VEGF properties and the role they may play in either the development or the cessation of growth of the tumor, but I’m not sure this explains what we see.
The message is that bevacizumab is a very exciting drug. This is an agent that will be in every Phase III study we develop or design in non-small cell lung cancer, and it should be widely available for patients with this disease. However, we need to be careful about some rather unique complications, including cavitation and hemoptysis, that we’re not accustomed to seeing in patients with non-small cell lung cancer. The other aspect of this drug is that it still does not apply to a substantial percentage of patients with this disease: patients with squamous cell carcinomas, those with cavitating lesions at presentation or who have hemoptysis of almost any degree at presentation. Corey Langer looked at his data from Fox-Chase and asked, “How many patients would not qualify for the ECOG trial?” The number they came up with was approximately 30 percent.
Nonprotocol first-line chemotherapy regimens
I almost always use carboplatin as opposed to cisplatin in the Stage IV population. I have used carboplatin with gemcitabine, docetaxel or paclitaxel. I have used carboplatin/vinorelbine in the past, but not recently. The specific choice is based on the toxicities of the chemotherapy regimen. I discuss the toxicities and issues of convenience with the patient because there are different schedules for carboplatin/gemcitabine. You have to come back for day eight, and then you have the advantage of less hair loss and other benefits, but hematologic toxicity may be somewhat more pronounced. Then you run into the taxane issues of neuropathy, myalgias, arthralgias, hair loss, etcetera. It may sound unusual, but I really make an effort to go in with an open mind. I almost never make that decision a priori, even though I may know the patient.
I have not yet utilized bevacizumab off study, but if I were to use it, I would probably be more reluctant to use it with carboplatin and gemcitabine than I would with the taxane regimens. The bleeding complications from the bevacizumab are not related to hematologic toxicity, but carbo-gemcitabine-related thrombocytopenia is obviously something you would like to avoid in a regimen that can cause bleeding.
Incorporating bevacizumab into adjuvant therapy
A couple of months ago, the leaders of the major cooperative groups met to discuss adjuvant trials. At that time, people were waiting for the ECOG data to be released, and it’s my impression that there will now be an adjuvant study with bevacizumab. As we move into the adjuvant setting with bevacizumab, we may not have such a restricted population because the tumor will be resected. So I believe the risk of bleeding, cavitation and other side effects will be much less. Off protocol, especially in the adjuvant setting, because we’re dealing with curable patients, the dogma is that an agent shouldn’t be utilized unless Phase III evidence exists. I abide by that dogma, but I have no doubts that we will be tempted in patients at high risk to use the bevacizumab in addition to the standard regimen that we use in the adjuvant setting. As long as an honest and frank discussion occurs with the patient about the potential complications, I think that is reasonable, but personally, I don’t think I will be bringing that topic up very often.
Combining bevacizumab with erlotinib
The ECOG study published in the JCO by Roy Herbst and Alan Sandler was very exciting, not just because of the results but because this is a proof of principle (Herbst 2005; [2.1]). They published data from a study evaluating the combination of bevacizumab and erlotinib in previously treated patients with non-small cell lung cancer. The study demonstrated a nice response rate and a median survival that was close to 12 months. Obviously, it’s a selected population and this is not the type of survival rate that we see for all of our patients, but it’s enough to justify taking this regimen ahead, and I believe the erlotinib/bevacizumab will be used in various settings. The idea that two different biologic agents can be combined means we can get away from standard chemotherapy completely and hopefully obtain the results they published. That is exciting.
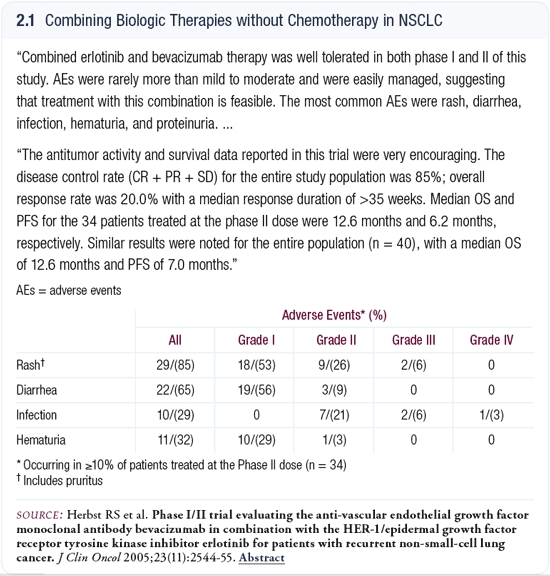
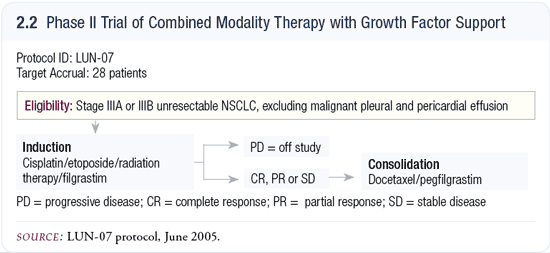
Chemotherapy and radiation therapy in Stage III disease
Our group is conducting a trial (2.2) based on the SWOG model using cisplatin and etoposide for two cycles during thoracic radiotherapy followed by three cycles of docetaxel. In the SWOG study S9504, which utilized this regimen, significant hematologic toxicity occurred, which mandated a substantial dose reduction (Gandara 2003). This was more important in the docetaxel consolidation, but it was also significant during the combined portion of the trial. We’re adding growth factors to that combined modality portion to see if we can minimize dose reduction and maintain dose intensity, which we believe is important in Stage III disease. Data from an old small cell trial that Paul Bunn published indicated that growth factors did abbreviate hematologic toxicity, but thrombocytopenia and pulmonary toxicity were more pronounced (Bunn 1995). Therefore, growth factors are not used with combined modality. We are evaluating this to see if it’s feasible and beneficial.
TRIBUTE trial: Chemotherapy with or without erlotinib
Vince Miller performed a subset analysis from the TRIBUTE trial (Miller 2004). They looked at the nonsmokers who received chemotherapy plus the TKI versus those who received chemotherapy only. The difference in median survival was one of the most impressive and overwhelming I have ever seen in a lung cancer study. In fact, when I first looked at the curves, it didn’t look like a non-small cell lung cancer curve. It was 22 versus 10 months. I’ve had the opportunity to apply the data to a couple of patients. My only question was whether I should have done the chemotherapy first, followed by a TKI after four cycles, or if I should have done it exactly the way it was done in the TRIBUTE trial, which was to start the three drugs at the same time.
Select publications
 |
Dr Lilenbaum is a Clinical Associate Professor of Medicine at the University of Miami School of Medicine and Director of the Thoracic Oncology Program at The Mount Sinai Comprehensive Cancer Center in Miami Beach, Florida. |
|
|

