|
You are here: Home: LCU 3 | 2005 : Alan B Sandler, MD
Alan B Sandler, MD |
EDITED COMMENTS |
 Clinical trials comparing carboplatin/paclitaxel with or without bevacizumab in patients with metastatic NSCLC Clinical trials comparing carboplatin/paclitaxel with or without bevacizumab in patients with metastatic NSCLC
Efficacy data
ECOG-E4599 was based on results from a randomized, Phase II, limited-institution study in which we participated. The trial enrolled 99 patients who were randomly assigned to paclitaxel/carboplatin alone versus paclitaxel/carboplatin with bevacizumab 7.5 mg/kg versus paclitaxel/carboplatin with bevacizumab 15 mg/kg every three weeks (Johnson 2004).
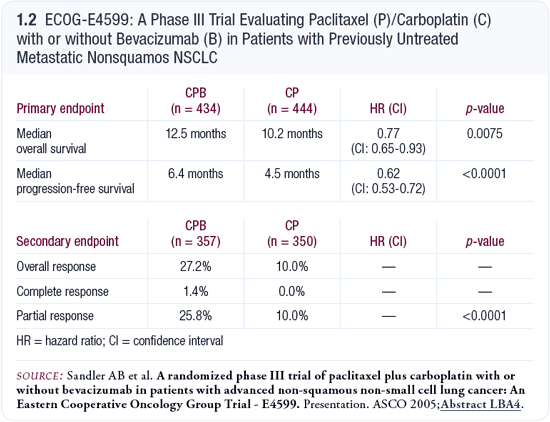
With the addition of bevacizumab, the results were interesting in terms of improvement in response rate, time to progression and survival. While only time to progression was statistically significant, the data suggest bevacizumab added some benefit to chemotherapy. ECOG-E4599 then evaluated carboplatin/ paclitaxel with or without bevacizumab, and the top-line data revealed that the median survival increased from 10.2 months to 12.6 months in patients receiving bevacizumab (Sandler 2005; [1.2]). A 25 percent improvement in survival is important, both statistically and clinically. I believe that as a result of the data, the new standard for patients who fit the criteria of ECOG-E4599 will be chemotherapy combined with bevacizumab.
Toxicity data
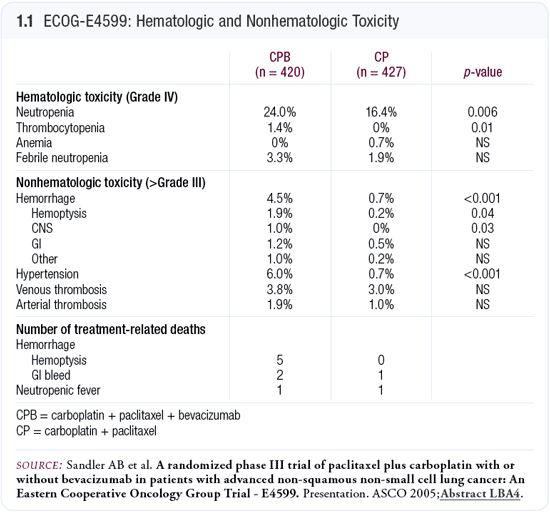
In the Phase II trial, issues arose with pulmonary hemorrhage and hemoptysis that seemed to be associated with squamous cell cancers. ECOG-E4599 excluded patients with these tumors, patients with brain metastases and patients on therapeutic anticoagulation. When we designed the Phase III trial, we paid particular attention to toxicity (Sandler 2005; [1.1]). As the protocol chair, I received toxicity data on a real-time basis, and the study was stopped after 112 patients for an interim analysis.
The interim data presented at ASCO in 2003 showed that while a difference occurred in the incidence of Grade V hemoptysis for patients on bevacizumab, it was not statistically significant and was lower than that seen in the Phase II trial (Gray 2003). The study was then amended to accrue approximately 840 patients in hopes of detecting a 25 percent difference in survival. The last patient was enrolled in April 2004.
Clinical impact
An interesting question raised by ECOG-E4599 is whether the FDA will approve bevacizumab in combination with only carboplatin/paclitaxel, with all platinbased chemotherapy or with all doublet chemotherapy. I doubt that combining it with other chemotherapies would be problematic, as bevacizumab is an antibody and is not metabolized by the liver. The only potential issue, given the concern for bleeding, would be combining it with an agent known to cause thrombocytopenia, although the thrombocytopenia associated with bevacizumab is more of a paper toxicity in that it doesn’t usually get below 50,000/μL.
Selecting adjuvant chemotherapy in the nonprotocol setting
In the adjuvant setting, I tend to use a cisplatin-based doublet such as cisplatin/gemcitabine, although I have used paclitaxel/carboplatin. Cisplatin/docetaxel is also reasonable, as is cisplatin/vinorelbine, which was studied in the French trial. We have a wide range of choices that can be utilized, and they appear to be somewhat equivalent. Adjuvant therapy for patients with Stage III disease remains controversial, so for the unresectable patients I’ve used cisplatin/etoposide with concurrent radiation therapy followed by docetaxel, as in the SWOG study. I like cisplatin/etoposide because I can administer it in full dose with radiation therapy. With weekly paclitaxel and weekly carboplatin, some of the data, such as from the CALGB study, were not as exciting as we had hoped. However, I still think that is a reasonable approach and have used that also. There remain a number of choices for adjuvant therapy, and without any head-to-head studies, we’re left with a lot of open-ended decisions.
Use of erlotinib in patients with metastatic disease
We now have essentially three approved agents in the second-line metastatic setting — docetaxel, pemetrexed and erlotinib — and I’m comfortable with each of these. Erlotinib is approved for second- and third-line therapy, and I’m content to use it in either setting. In patients who have received and done well with front-line chemotherapy, it’s reasonable to consider chemotherapy again, although I would use a different agent. In patients who have not fared well with chemotherapy in the front-line setting (either they progressed or were unable to tolerate it), I tend to use erlotinib. In patients with poor performance status — maybe a PS 2+, someone who’s not interested in chemotherapy, an older octogenarian-plus or a nonsmoking, Asian female with adenocarcinoma — it’s tempting to consider first-line therapy with erlotinib. However, chemotherapy has a more proven track record in that setting, so I still prefer to use chemotherapy.
The question is whether erlotinib adds to chemotherapy given concurrently or sequentially. Vince Miller’s data from the TRIBUTE trial showed a survival advantage for chemotherapy and erlotinib versus chemotherapy alone in nonsmokers. However, the curve suggests that the improvement is seen in the first few months after the chemotherapy is finished, whereas when they’re given together they seem similar. For that reason, I have on occasion used chemotherapy followed by maintenance therapy, with a tyrosine kinase inhibitor, particularly in an Asian patient with adenocarcinoma who never smoked.
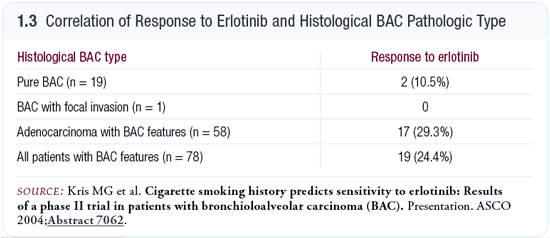
The data on bronchioloalveolar carcinoma (BAC) from Adi Gazdar at the University of Texas Southwestern show that the pure BACs, which represent a small minority, do not seem to have the EGFR mutation (Shigematsu 2005). However, adenocarcinomas with varying degrees of BAC do have the mutation, and those patients tend to do very well. So that’s another group in which erlotinib alone could be beneficial. We participated in a Memorial Sloan-Kettering study of erlotinib in first- and second-line treatment of patients with some degree of BAC, and the response rates were around 26 percent.
Phase I/II trial of bevacizumab in combination with erlotinib
Roy Herbst and I conducted a Phase I/II trial at MD Anderson and Vanderbilt, respectively, evaluating the combination of bevacizumab and erlotinib in patients with previously treated non-small cell lung cancer (Herbst 2005). We excluded patients with squamous cell tumors and brain metastases. It wasn’t an official Phase I trial in that we didn’t push for maximum tolerated dose. Rather, we wanted to evaluate whether we could reach the full dose of each single agent when combined, and we did. We treated 40 patients overall, and 34 patients received the Phase II doses of erlotinib 150 mg and bevacizumab 15 mg/kg.
In this study, the response rate was 20 percent with a median survival of 12.6 months, and time to progression was over six months. Although we didn’t know at the time that it was a good thing to do, we now recognize that the study had an enriched population for erlotinib because we excluded squamous cell tumors. What was interesting is that we saw responses in patients whom we did not expect to respond to erlotinib alone, such as smokers, males and African-American males. In addition, 65 percent of patients experienced stable disease, which is double what one would expect.
Randomized trials of combination chemotherapy in SCLC
Data were published in 2002 from a Japanese trial comparing etoposide/cisplatin versus irinotecan/cisplatin in 154 previously untreated patients with extensive SCLC (Noda 2002; [1.3]). The data showed the irinotecan combination significantly improved survival, and the trial was closed early because of the survival advantage. The patients who received the irinotecan-containing regimen had a median overall survival of approximately 12.75 months versus approximately 9.3 months for the patients on the standard etoposide/cisplatin regimen. We then conducted a larger, Phase III study of etoposide/cisplatin versus irinotecan/cisplatin with 330 patients, using a three-week schedule for cisplatin/irinotecan rather than the four-week schedule used in the Japanese trial. Our study was designed to detect a survival advantage of 33 percent with irinotecan, and we expect to have the data by ASCO 2005.
Select publications
 |
Dr Sandler is an Associate Professor of Medicine, the Medical Director of Thoracic Oncology and the Director of the Vanderbilt-Ingram Cancer Center Affiliate Network Program at the Vanderbilt University Medical Center Division of Hematology/Oncology in Nashville, Tennessee. |
|
|

