
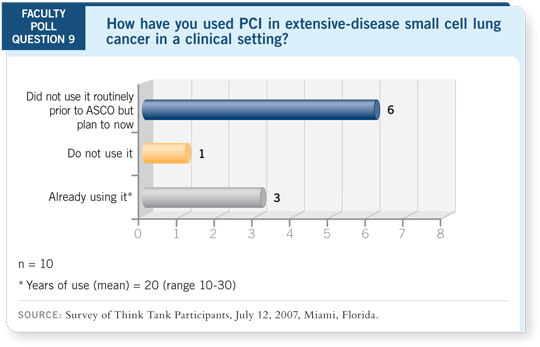
Select Excerpts from the Discussion
Tracks 1-2
 DR LOVE:
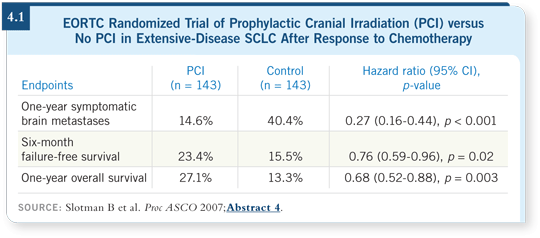
DR LOVE: Wally, can you discuss the implications of the EORTC-08993
trial that evaluated the impact of PCI on the reduction of symptomatic
brain metastases for patients with extensive-disease SCLC who responded
to chemotherapy (Slotman 2007; [4.1])?
 DR CURRAN: My concern with this study is that the patients did not undergo
meticulous restaging, including brain scans at baseline. At the recent RTOG
meeting, we discussed the possibility that these patients were being treated for
subclinical brain metastases. I am not comfortable with a randomized trial in
which you don’t evaluate the brain before you treat it. I have to assume there’s
a 10 to 25 percent risk of subclinical disease. Also, their definition of chemotherapy
response to continue on to the randomization was not particularly
rigorous.
DR CURRAN: My concern with this study is that the patients did not undergo
meticulous restaging, including brain scans at baseline. At the recent RTOG
meeting, we discussed the possibility that these patients were being treated for
subclinical brain metastases. I am not comfortable with a randomized trial in
which you don’t evaluate the brain before you treat it. I have to assume there’s
a 10 to 25 percent risk of subclinical disease. Also, their definition of chemotherapy
response to continue on to the randomization was not particularly
rigorous.
The positive results are an interesting observation, but it is not up to American clinical research standards. The magnitude of survival benefit is puzzling.
How can the survival benefit be greater than in limited disease?
 DR HANNA: I agree with Wally about the criticism that they should have
obtained baseline brain imaging studies on all patients. However, if the patient
met certain criteria that raised suspicion of brain metastases, they did require
baseline brain imaging. They didn’t tell us how many patients had undergone
baseline brain imaging, which would have been useful. There was also
an imbalance in the proportion of patients who had other sites of metastases,
presumably liver and adrenal, which was worse in the observation arm.
DR HANNA: I agree with Wally about the criticism that they should have
obtained baseline brain imaging studies on all patients. However, if the patient
met certain criteria that raised suspicion of brain metastases, they did require
baseline brain imaging. They didn’t tell us how many patients had undergone
baseline brain imaging, which would have been useful. There was also
an imbalance in the proportion of patients who had other sites of metastases,
presumably liver and adrenal, which was worse in the observation arm.
The problem I have isn’t that the study is not provocative and we probably
ought to be doing it for some patients; it’s that the author’s conclusion was PCI
is now the standard practice for all patients with extensive-stage SCLC who
are responding. If you have a patient with liver and adrenal metastases that has
some response to initial chemotherapy, it’s ridiculous to use PCI.
 DR LOVE: Describe the patient whom you would treat with PCI.
DR LOVE: Describe the patient whom you would treat with PCI.
 DR HANNA: I would use it with the patient who is free of bulky liver, adrenal
or bone metastases or the patient who has an excellent response to chemotherapy
and based on clinical intuition is going to survive for a while. Those
are the patients who will suffer from symptomatic brain metastases.
DR HANNA: I would use it with the patient who is free of bulky liver, adrenal
or bone metastases or the patient who has an excellent response to chemotherapy
and based on clinical intuition is going to survive for a while. Those
are the patients who will suffer from symptomatic brain metastases.
 DR GRECO: I believe selected patients can benefit, and this study would support
that. Most studies — even the large trials — don’t tell us about individual
patients. You use that information in the context of the patient you see in your
office that day. You don’t just say, “This study showed a survival benefit, so I’m
going to use this therapy for every patient with extensive-stage SCLC.”
DR GRECO: I believe selected patients can benefit, and this study would support
that. Most studies — even the large trials — don’t tell us about individual
patients. You use that information in the context of the patient you see in your
office that day. You don’t just say, “This study showed a survival benefit, so I’m
going to use this therapy for every patient with extensive-stage SCLC.”
Select Publication

