
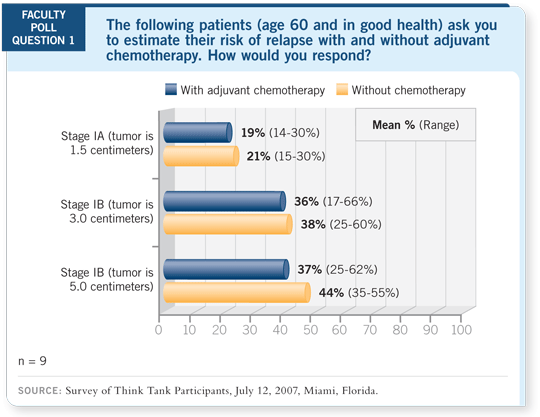
Select Excerpts from the Discussion
Track 15
 DR LOVE:
DR LOVE: Ed, how do you approach the choice of an adjuvant chemotherapy
regimen?
 DR KIM: I generally use cisplatin-based therapy. I use cisplatin/docetaxel 60 to
70 percent of the time and cisplatin/vinorelbine 30 to 40 percent of the time.
DR KIM: I generally use cisplatin-based therapy. I use cisplatin/docetaxel 60 to
70 percent of the time and cisplatin/vinorelbine 30 to 40 percent of the time.
 DR LOVE: Nasser?
DR LOVE: Nasser?
 DR HANNA: I use docetaxel/cisplatin. The logic behind that is docetaxel is
a slightly superior drug compared to vinorelbine in the metastatic setting,
and vinorelbine is the drug for which we have the most data in the adjuvant
setting. I extrapolate that docetaxel will be more effective in the adjuvant setting. However, I have no qualms with colleagues using cisplatin/vinorelbine
or other regimens from the trials.
DR HANNA: I use docetaxel/cisplatin. The logic behind that is docetaxel is
a slightly superior drug compared to vinorelbine in the metastatic setting,
and vinorelbine is the drug for which we have the most data in the adjuvant
setting. I extrapolate that docetaxel will be more effective in the adjuvant setting. However, I have no qualms with colleagues using cisplatin/vinorelbine
or other regimens from the trials.
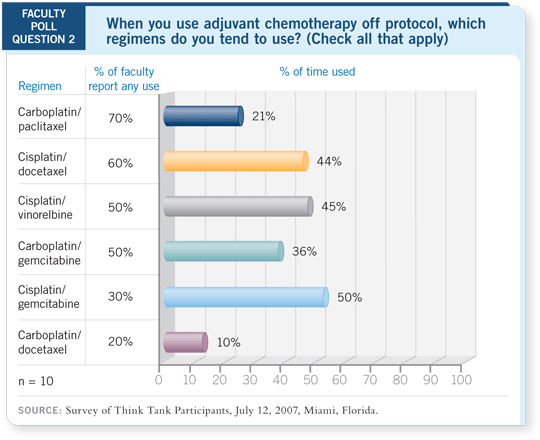
 DR LYNCH: I agree with Nasser. I answered the question as 90 percent cisplatin/docetaxel and 10 percent cisplatin/gemcitabine. However, if I see a 69-year-old patient six weeks postoperatively with a creatinine level of 1.7 mg/dL
who doesn’t look so great, I would likely use carboplatin/paclitaxel.
DR LYNCH: I agree with Nasser. I answered the question as 90 percent cisplatin/docetaxel and 10 percent cisplatin/gemcitabine. However, if I see a 69-year-old patient six weeks postoperatively with a creatinine level of 1.7 mg/dL
who doesn’t look so great, I would likely use carboplatin/paclitaxel.
Tracks 21-22
 DR LOVE:
DR LOVE: Joan, can you discuss ECOG-E1505, which randomly assigns
patients to adjuvant therapy with a cisplatin-based regimen with or
without bevacizumab (1.1)?
 DR SCHILLER: ECOG-E1505 is a Phase III trial for patients with selected
Stage IB to IIIA NSCLC, who will be randomly assigned to four cycles
of chemotherapy versus four cycles of chemotherapy and up to one year of
bevacizumab. To some degree, the chemotherapy will be “dealer’s choice.”
The referring physician can choose among cisplatin/gemcitabine, cisplatin/docetaxel and cisplatin/vinorelbine.
DR SCHILLER: ECOG-E1505 is a Phase III trial for patients with selected
Stage IB to IIIA NSCLC, who will be randomly assigned to four cycles
of chemotherapy versus four cycles of chemotherapy and up to one year of
bevacizumab. To some degree, the chemotherapy will be “dealer’s choice.”
The referring physician can choose among cisplatin/gemcitabine, cisplatin/docetaxel and cisplatin/vinorelbine.
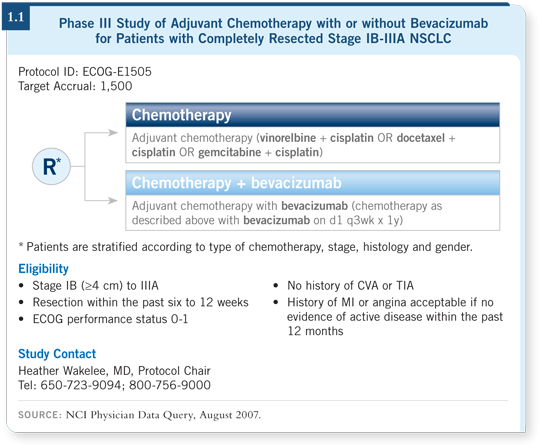
Eligible patients will have Stage IB to IIIA disease, with eligible IB tumors
measuring greater than four centimeters in size. The reason for that is based on
a subset analysis CALGB conducted of their adjuvant study, in which patients
with larger Stage IB tumors were the ones who seemed to benefit (Strauss
2006). We’ll apply the typical bevacizumab exclusion criteria. Patients will be
allowed to have had squamous cell carcinoma, however, because the disease
will be removed. It is hoped that the histology will not be important if the
tumor is not there.
 DR GRECO: I believe the scientific aspects of the study are good, but we see a
lot of arbitrary thinking about which adjuvant regimens we should use. People
have strong feelings about cisplatin, and although I prefer to be more lenient
with the type of chemotherapy allowed, many purists are designing the studies.
DR GRECO: I believe the scientific aspects of the study are good, but we see a
lot of arbitrary thinking about which adjuvant regimens we should use. People
have strong feelings about cisplatin, and although I prefer to be more lenient
with the type of chemotherapy allowed, many purists are designing the studies.
 DR SANDLER: : I agree. The study was originally for all patients with Stage IB to
IIIA disease. Then the CALGB update reported on this 4-cm concept, and the
NCI was adamant that we use the 4-cm cutoff. So we’re using the 4-cm cutoff
based on retrospective data from the CALGB-9633 study, which used paclitaxel/carboplatin (Strauss 2006), but they won’t allow paclitaxel/carboplatin in the
E1505 study, which has been the only regimen to report survival data with
bevacizumab (Sandler 2006).
DR SANDLER: : I agree. The study was originally for all patients with Stage IB to
IIIA disease. Then the CALGB update reported on this 4-cm concept, and the
NCI was adamant that we use the 4-cm cutoff. So we’re using the 4-cm cutoff
based on retrospective data from the CALGB-9633 study, which used paclitaxel/carboplatin (Strauss 2006), but they won’t allow paclitaxel/carboplatin in the
E1505 study, which has been the only regimen to report survival data with
bevacizumab (Sandler 2006).
 DR SOCINSKI: I believe patients will balk not at the randomization between
chemotherapy with or without bevacizumab but at receiving bevacizumab for a
year. I speak with a lot of patients about adjuvant therapy — it usually is three or
four cycles, and then they’re done. Now you have the patient who is postthoracotomy
thinking about treatment that is either nine weeks or 12 months.
DR SOCINSKI: I believe patients will balk not at the randomization between
chemotherapy with or without bevacizumab but at receiving bevacizumab for a
year. I speak with a lot of patients about adjuvant therapy — it usually is three or
four cycles, and then they’re done. Now you have the patient who is postthoracotomy
thinking about treatment that is either nine weeks or 12 months.
Track 51
 DR LOVE:
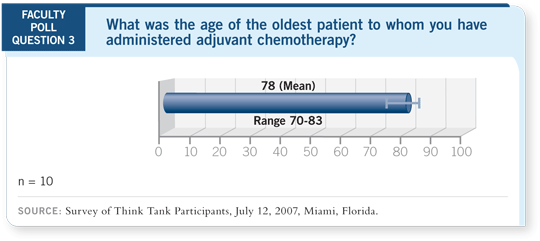
DR LOVE: Tom, our faculty has treated patients between 70 and 83 years
of age with adjuvant chemotherapy. What are your thoughts?
 DR LYNCH: I believe that is a reasonable range. I don’t believe it’s wrong if
you have an 83-year-old woman without other medical problems. It’s difficult
to imagine she has lung cancer with no other medical problems, but if that’s
the case, it’s reasonable to treat someone regardless of age. The catch is that
there are very few patients who fit this description exactly. In general, we do
consider age as a factor. Early eighties is probably as high as one should go.
DR LYNCH: I believe that is a reasonable range. I don’t believe it’s wrong if
you have an 83-year-old woman without other medical problems. It’s difficult
to imagine she has lung cancer with no other medical problems, but if that’s
the case, it’s reasonable to treat someone regardless of age. The catch is that
there are very few patients who fit this description exactly. In general, we do
consider age as a factor. Early eighties is probably as high as one should go.
 DR SOCINSKI: When you use adjuvant therapy in older patients, what are you
using? My practice has been to use cisplatin for all patients when I can, but
we know cisplatin is a more toxic drug in the elderly. I was the one who used
adjuvant therapy in the 83-year-old, and I used carboplatin/paclitaxel.
DR SOCINSKI: When you use adjuvant therapy in older patients, what are you
using? My practice has been to use cisplatin for all patients when I can, but
we know cisplatin is a more toxic drug in the elderly. I was the one who used
adjuvant therapy in the 83-year-old, and I used carboplatin/paclitaxel.
Select Publications

