
 |
|||||||

| Tracks 1-21 | ||||||||||||||||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Track 3, 5
![]() DR LOVE: Can you discuss the assays for molecular markers that have
been studied for predicting response to the EGFR TKIs?
DR LOVE: Can you discuss the assays for molecular markers that have
been studied for predicting response to the EGFR TKIs?
![]() DR RIELY: A number of retrospective evaluations have been conducted of
tumor sets from large randomized trials evaluating EGFR overexpression
with immunohistochemistry (IHC) and FISH in addition to evaluating EGFR
mutations. It’s complicated, but I would break it down to an issue of sensitivity
and specificity. Patients with EGFR IHC-positive tumors represent the
majority of lung cancer patients, and these patients may or may not respond to
EGFR TKIs. IHC does not serve as much of an enrichment for patients likely
to respond to erlotinib.
DR RIELY: A number of retrospective evaluations have been conducted of
tumor sets from large randomized trials evaluating EGFR overexpression
with immunohistochemistry (IHC) and FISH in addition to evaluating EGFR
mutations. It’s complicated, but I would break it down to an issue of sensitivity
and specificity. Patients with EGFR IHC-positive tumors represent the
majority of lung cancer patients, and these patients may or may not respond to
EGFR TKIs. IHC does not serve as much of an enrichment for patients likely
to respond to erlotinib.
Fewer patients have true FISH-positive tumors. Among unselected patients, the overall response rate with erlotinib is approximately nine percent. If you identify those patients with FISH-positive tumors, the response rate jumps significantly and is closer to 40 percent. Approximately 10 percent of patients with lung cancer have EGFR mutations, but the response rate for those tumors is closer to 80 percent (van Zandwijk 2007).
The EGFR mutation represents the most specific predictor of response to EGFR TKIs, whereas EGFR overexpression as determined by IHC and FISH describes a larger number of patients, but those patients are less likely to respond to erlotinib (Jackman 2008; Zhu 2008).
Tracks 6, 8
![]() DR LOVE: What is your approach to treatment for a patient with an
EGFR mutation in the adjuvant setting? Do you offer erlotinib?
DR LOVE: What is your approach to treatment for a patient with an
EGFR mutation in the adjuvant setting? Do you offer erlotinib?
![]() DR RIELY: It is difficult to argue against the idea of using erlotinib — with
a response rate of more than 80 percent — for a patient with an EGFR
mutation. However, it’s unclear whether the EGFR mutation is a predictive
factor or a prognostic factor. Retrospective analyses of patients with EGFR
mutations have shown that they fare relatively well after resection in comparison
to those with EGFR wild-type tumors, and these patients may go on to
fare better overall, whether we administer erlotinib or not.
DR RIELY: It is difficult to argue against the idea of using erlotinib — with
a response rate of more than 80 percent — for a patient with an EGFR
mutation. However, it’s unclear whether the EGFR mutation is a predictive
factor or a prognostic factor. Retrospective analyses of patients with EGFR
mutations have shown that they fare relatively well after resection in comparison
to those with EGFR wild-type tumors, and these patients may go on to
fare better overall, whether we administer erlotinib or not.
At the same time, lung cancer is a difficult diagnosis. For a patient with early-stage resected lung cancer who has a known EGFR mutation, I explain that no data exist to demonstrate improvements in overall survival with erlotinib, but my scientific estimation is that it will.
![]() DR LOVE: For patients with metastatic NSCLC who have the EGFR
mutation, do you recommend erlotinib up front?
DR LOVE: For patients with metastatic NSCLC who have the EGFR
mutation, do you recommend erlotinib up front?
![]() DR RIELY: You can make a perfectly reasonable argument to start patients
with known EGFR mutations or with clinical characteristics that are likely to
be associated with mutations — such as never smokers with adenocarcinomas
— on erlotinib monotherapy, which is what I generally do off protocol.
DR RIELY: You can make a perfectly reasonable argument to start patients
with known EGFR mutations or with clinical characteristics that are likely to
be associated with mutations — such as never smokers with adenocarcinomas
— on erlotinib monotherapy, which is what I generally do off protocol.
Track 9
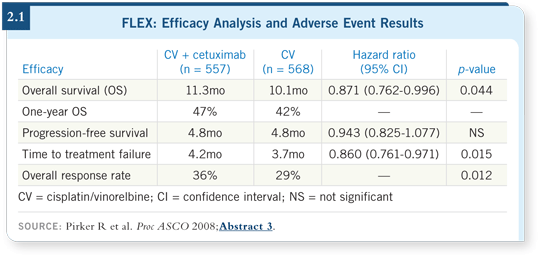
![]() DR LOVE: Can you summarize the FLEX trial results that were presented
at ASCO (2.1)?
DR LOVE: Can you summarize the FLEX trial results that were presented
at ASCO (2.1)?
![]() DR RIELY: In the FLEX study, patients were randomly assigned to receive
either cisplatin/vinorelbine alone or cisplatin/vinorelbine with cetuximab
(Pirker 2008). Patients received cetuximab on a weekly basis, and after six
cycles of chemotherapy, those patients who were on cetuximab continued it as
maintenance therapy.
DR RIELY: In the FLEX study, patients were randomly assigned to receive
either cisplatin/vinorelbine alone or cisplatin/vinorelbine with cetuximab
(Pirker 2008). Patients received cetuximab on a weekly basis, and after six
cycles of chemotherapy, those patients who were on cetuximab continued it as
maintenance therapy.
Approximately a one-month improvement in overall survival was observed, without a marked difference in progression-free survival, for patients treated with cetuximab. Subset analysis revealed that cetuximab was equally efficacious in squamous cell tumors and adenocarcinomas. As such, chemotherapy with cetuximab would be a reasonable choice for patients with squamous cell tumors, for which we are unable to use bevacizumab safely.
Tracks 12-13
![]() DR LOVE: Do you think cetuximab has a positive risk-benefit ratio for
patients with advanced squamous cell NSCLC?
DR LOVE: Do you think cetuximab has a positive risk-benefit ratio for
patients with advanced squamous cell NSCLC?
![]() DR RIELY: It’s reasonable to consider cetuximab for patients with squamous
cell tumors. The side effects of cetuximab are real, including the rash and the
side effect that is rarely talked about, which is weekly therapy. Patients must
come to the doctor’s office once a week for the first six cycles of therapy,
which is a burden for a patient with Stage IV NSCLC.
DR RIELY: It’s reasonable to consider cetuximab for patients with squamous
cell tumors. The side effects of cetuximab are real, including the rash and the
side effect that is rarely talked about, which is weekly therapy. Patients must
come to the doctor’s office once a week for the first six cycles of therapy,
which is a burden for a patient with Stage IV NSCLC.
![]() DR LOVE: How do you approach patients with adenocarcinomas who meet
the entry criteria for ECOG-E4599, which evaluated carboplatin/paclitaxel
and bevacizumab?
DR LOVE: How do you approach patients with adenocarcinomas who meet
the entry criteria for ECOG-E4599, which evaluated carboplatin/paclitaxel
and bevacizumab?
![]() DR RIELY: Chemotherapy with cetuximab is an option, but carboplatin/paclitaxel
and bevacizumab led to a two-month improvement in overall survival,
compared to somewhat less improvement in overall survival in the FLEX
trial. Therefore, it’s reasonable to consider bevacizumab as probably superior,
acknowledging the caveats about cross-trial comparisons. Additionally, we
have much greater experience administering bevacizumab for a large number
of patients.
DR RIELY: Chemotherapy with cetuximab is an option, but carboplatin/paclitaxel
and bevacizumab led to a two-month improvement in overall survival,
compared to somewhat less improvement in overall survival in the FLEX
trial. Therefore, it’s reasonable to consider bevacizumab as probably superior,
acknowledging the caveats about cross-trial comparisons. Additionally, we
have much greater experience administering bevacizumab for a large number
of patients.
Track 14
![]() DR LOVE: How do you treat the patient with an EGFR mutation who
responds to erlotinib and then experiences disease progression?
DR LOVE: How do you treat the patient with an EGFR mutation who
responds to erlotinib and then experiences disease progression?
![]() DR RIELY: This is an important question, to which we don’t have an answer.
The direct analogy is a patient with HER2-positive breast cancer who
responds to trastuzumab and then develops progressive disease. The standard
approach to those patients without evidence from a randomized trial is to
continue trastuzumab and add chemotherapy.
DR RIELY: This is an important question, to which we don’t have an answer.
The direct analogy is a patient with HER2-positive breast cancer who
responds to trastuzumab and then develops progressive disease. The standard
approach to those patients without evidence from a randomized trial is to
continue trastuzumab and add chemotherapy.
We investigated this situation with a relatively small study, in which we took 10 patients with what we defined as acquired resistance to erlotinib or gefitinib (Riely 2007). Those patients had all been treated with erlotinib or gefitinib for more than six months and all had responded to therapy. If we couldn’t document a response to therapy, we verified that the tumor had an EGFR mutation.
So we took this relatively select group of patients and performed scans on them. We discontinued therapy at disease progression and rescanned them. As you would expect, most of the tumors grew. FDG avidity also rose on the PET scan because disease was progressing in all cases beforehand, so it’s reasonable to presume that it would continue to progress off treatment. We restarted gefitinib or erlotinib for another three weeks and then reassessed. Somewhat surprisingly, all of the tumors stabilized in their growth rate and FDG uptake on PET scan. These data suggest that these patients are continuing to benefit from erlotinib therapy, so our standard treatment is to continue erlotinib and add chemotherapy.
Track 17
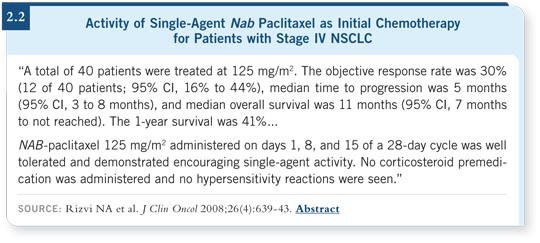
![]() DR LOVE: Can you discuss the study you were involved with evaluating
nanoparticle albumin-bound (nab) paclitaxel in patients with previously
untreated NSCLC (Rizvi 2008)?
DR LOVE: Can you discuss the study you were involved with evaluating
nanoparticle albumin-bound (nab) paclitaxel in patients with previously
untreated NSCLC (Rizvi 2008)?
![]() DR RIELY: This was a Phase I/II trial. After initially identifying the
maximum tolerated dose (MTD), we treated patients with the MTD of nab paclitaxel on a weekly administration schedule. The response rate with single-agent
nab paclitaxel was 30 percent, and the overall survival was acceptable for
up-front treatment in NSCLC (Rizvi 2008; [2.2]).
DR RIELY: This was a Phase I/II trial. After initially identifying the
maximum tolerated dose (MTD), we treated patients with the MTD of nab paclitaxel on a weekly administration schedule. The response rate with single-agent
nab paclitaxel was 30 percent, and the overall survival was acceptable for
up-front treatment in NSCLC (Rizvi 2008; [2.2]).
Nab paclitaxel is clearly an effective drug, and compared to conventional taxanes, its benefits are apparent, such as the absence of hypersensitivity reactions in patients who are receiving the taxane without steroid premedication.
| Table of Contents | Top of Page |
EDITOR
Neil Love, MD
INTERVIEWS
David Jablons, MD
- Select publications
Gregory J Riely, MD, PhD
- Select publications
Alan B Sandler, MD
- Select publications
David S Ettinger, MD
- Select publications
Lung Cancer Update:
A CME Audio Series and Activity