
 |
|||||||

| Tracks 1-17 | ||||||||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Tracks 1-2
![]() DR LOVE: How do you approach the selection of chemotherapy in the
adjuvant setting?
DR LOVE: How do you approach the selection of chemotherapy in the
adjuvant setting?
![]() DR WOZNIAK: Whether to use cisplatin or carboplatin is a big question. In practice, I believe most physicians prefer using carboplatin because it’s easier.
All the evidence, even in the advanced-disease setting, indicates that cisplatin is
likely more effective. It may not make a difference for patients with metastatic
disease, but it may make a difference in the adjuvant setting. Generally, I use
cisplatin whenever possible, combined with either vinorelbine or docetaxel.
Most of the trials, including the Canadian trial (Winton 2005) and the ANITA
trial (Douillard 2005, 2006), used vinorelbine. In IALT, approximately 25
percent of the patients received vinorelbine and approximately half of the
patients received etoposide (Arriagada 2004).
DR WOZNIAK: Whether to use cisplatin or carboplatin is a big question. In practice, I believe most physicians prefer using carboplatin because it’s easier.
All the evidence, even in the advanced-disease setting, indicates that cisplatin is
likely more effective. It may not make a difference for patients with metastatic
disease, but it may make a difference in the adjuvant setting. Generally, I use
cisplatin whenever possible, combined with either vinorelbine or docetaxel.
Most of the trials, including the Canadian trial (Winton 2005) and the ANITA
trial (Douillard 2005, 2006), used vinorelbine. In IALT, approximately 25
percent of the patients received vinorelbine and approximately half of the
patients received etoposide (Arriagada 2004).
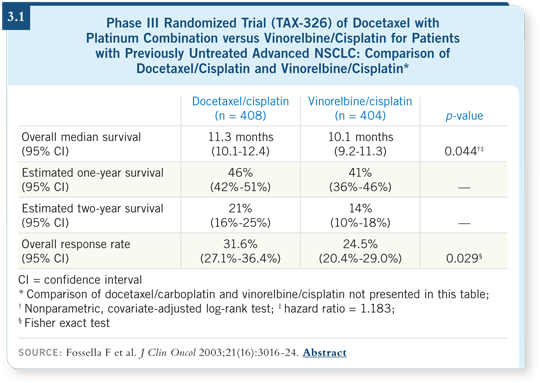
In advanced disease, TAX-326 compared cisplatin/vinorelbine to cisplatin/docetaxel and carboplatin/docetaxel. The cisplatin/docetaxel arm was better in that trial (Fossella 2003; [3.1]), which is why many people use it as adjuvant treatment.
Track 6, 8
![]() DR LOVE: Can you discuss the SWOG-S0533 study for patients with
unresectable Stage III NSCLC?
DR LOVE: Can you discuss the SWOG-S0533 study for patients with
unresectable Stage III NSCLC?
![]() DR WOZNIAK: It is still in the early stages of accrual. The goal of the trial is to
offer bevacizumab to these patients. Not a lot is known about the combination
of chemotherapy, radiation and bevacizumab. So the trial has two groups of
patients and three cohorts of treatment (3.2).
DR WOZNIAK: It is still in the early stages of accrual. The goal of the trial is to
offer bevacizumab to these patients. Not a lot is known about the combination
of chemotherapy, radiation and bevacizumab. So the trial has two groups of
patients and three cohorts of treatment (3.2).

One of the groups of patients will be considered at low risk with regard to hemoptysis associated with bevacizumab, and the other group will be considered at high risk. High risk is defined by predominantly squamous histology, a history of hemoptysis or a tumor that is fairly central, near a major blood vessel or with some cavitation. The low-risk and the high-risk groups will accrue independently.
The first cohort of patients will receive cisplatin/etoposide and radiation therapy. After a break, they will receive bevacizumab with docetaxel consolidation. If the side effects are acceptable in the high-risk and low-risk groups, then we move to the second cohort. It is similar to a Phase I study.
![]() DR LOVE: In the second cohort, will you add bevacizumab during chemoradiation
therapy?
DR LOVE: In the second cohort, will you add bevacizumab during chemoradiation
therapy?
![]() DR WOZNIAK: Yes, in the midst of it. Then, if we reach the third cohort, bevacizumab will be administered at the beginning of chemoradiation therapy.
We have another trial evaluating cisplatin/pemetrexed with radiation therapy
in Stage III disease. This trial was designed before the HOG trial results were
available (Hanna 2007), and we’re using docetaxel consolidation. Pemetrexed
is another drug that can be administered at full doses with radiation therapy.
I believe it is an up-and-coming drug in lung cancer and that it may be the
drug to use in Stage III disease. We decided to keep docetaxel consolidation
because a different chemotherapy agent is administered with the radiation
therapy, and this may be good.
DR WOZNIAK: Yes, in the midst of it. Then, if we reach the third cohort, bevacizumab will be administered at the beginning of chemoradiation therapy.
We have another trial evaluating cisplatin/pemetrexed with radiation therapy
in Stage III disease. This trial was designed before the HOG trial results were
available (Hanna 2007), and we’re using docetaxel consolidation. Pemetrexed
is another drug that can be administered at full doses with radiation therapy.
I believe it is an up-and-coming drug in lung cancer and that it may be the
drug to use in Stage III disease. We decided to keep docetaxel consolidation
because a different chemotherapy agent is administered with the radiation
therapy, and this may be good.
![]() DR LOVE: What data do we have with pemetrexed in combination with
chemoradiation therapy in Stage III disease?
DR LOVE: What data do we have with pemetrexed in combination with
chemoradiation therapy in Stage III disease?
![]() DR WOZNIAK: Studies are ongoing. Our trial is new, but in the patients we’ve
treated, it’s been tolerable. I can’t tell you anything about survival because
we don’t know that yet. Pemetrexed is well tolerated when administered in
concurrence with radiation therapy. Patients are generally able to get through
the entire treatment without dose reductions. Cisplatin/etoposide/radiation
therapy is also well tolerated. The question is which is the better combination.
DR WOZNIAK: Studies are ongoing. Our trial is new, but in the patients we’ve
treated, it’s been tolerable. I can’t tell you anything about survival because
we don’t know that yet. Pemetrexed is well tolerated when administered in
concurrence with radiation therapy. Patients are generally able to get through
the entire treatment without dose reductions. Cisplatin/etoposide/radiation
therapy is also well tolerated. The question is which is the better combination.
Track 13
![]() DR LOVE: What is your treatment algorithm for metastatic disease in a
nonsmoker or a nonsmoker with EGFR-positive disease?
DR LOVE: What is your treatment algorithm for metastatic disease in a
nonsmoker or a nonsmoker with EGFR-positive disease?
![]() DR WOZNIAK: The only factor I consider is nonsmoking status because that
seems to have the strongest support for using an EGFR inhibitor. In the clinical
setting, I believe the standard approach up front is still systemic chemotherapy.
As first-line therapy, I use carboplatin/paclitaxel or carboplatin/gemcitabine.
In terms of what to do with the never smoker — someone who’s smoked
fewer than 100 cigarettes in his or her lifetime — I will discuss the option of
erlotinib. If any patients were going to respond, they would be in that particular
group. The majority of patients who dramatically respond to these drugs
generally do so within the first four weeks of treatment. So if someone would
like that option, I don’t believe there’s anything wrong with it.
DR WOZNIAK: The only factor I consider is nonsmoking status because that
seems to have the strongest support for using an EGFR inhibitor. In the clinical
setting, I believe the standard approach up front is still systemic chemotherapy.
As first-line therapy, I use carboplatin/paclitaxel or carboplatin/gemcitabine.
In terms of what to do with the never smoker — someone who’s smoked
fewer than 100 cigarettes in his or her lifetime — I will discuss the option of
erlotinib. If any patients were going to respond, they would be in that particular
group. The majority of patients who dramatically respond to these drugs
generally do so within the first four weeks of treatment. So if someone would
like that option, I don’t believe there’s anything wrong with it.
![]() DR LOVE: Would you consider it as first-line therapy?
DR LOVE: Would you consider it as first-line therapy?
![]() DR WOZNIAK: For a never smoker, I would.
DR WOZNIAK: For a never smoker, I would.
Track 15
![]() DR LOVE: Can you describe the two SWOG studies that are evaluating
the combination of erlotinib and bevacizumab?
DR LOVE: Can you describe the two SWOG studies that are evaluating
the combination of erlotinib and bevacizumab?
![]() DR WOZNIAK: These trials will study patients who we perceive will benefit
more from erlotinib. Patients will receive the combination of erlotinib and
bevacizumab, which appeared promising in other clinical trials (Groen 2007;
Herbst 2005, 2007). We will also evaluate biologic correlates — mutations, EGFR expression and FISH analysis — that will be important in order to
determine whether characteristics of the tumor predict response.
DR WOZNIAK: These trials will study patients who we perceive will benefit
more from erlotinib. Patients will receive the combination of erlotinib and
bevacizumab, which appeared promising in other clinical trials (Groen 2007;
Herbst 2005, 2007). We will also evaluate biologic correlates — mutations, EGFR expression and FISH analysis — that will be important in order to
determine whether characteristics of the tumor predict response.
![]() DR LOVE: Are you using erlotinib/bevacizumab off study?
DR LOVE: Are you using erlotinib/bevacizumab off study?
![]() DR WOZNIAK: Not currently. We are participating in a Phase III study evaluating
erlotinib with bevacizumab or placebo as second-line treatment. I would
like to see the results of that study first.
DR WOZNIAK: Not currently. We are participating in a Phase III study evaluating
erlotinib with bevacizumab or placebo as second-line treatment. I would
like to see the results of that study first.
![]() DR LOVE: If a patient who is a never smoker, has the EGFR mutation or has
FISH-positive disease responds well to chemotherapy and bevacizumab, some
physicians will discontinue chemotherapy and continue treatment with bevacizumab.
At some point would you add erlotinib to the bevacizumab?
DR LOVE: If a patient who is a never smoker, has the EGFR mutation or has
FISH-positive disease responds well to chemotherapy and bevacizumab, some
physicians will discontinue chemotherapy and continue treatment with bevacizumab.
At some point would you add erlotinib to the bevacizumab?
![]() DR WOZNIAK: I like to see evidence before combining certain agents, but I
suspect that for that patient, even I might be willing to combine erlotinib and
bevacizumab.
DR WOZNIAK: I like to see evidence before combining certain agents, but I
suspect that for that patient, even I might be willing to combine erlotinib and
bevacizumab.
| Table of Contents | Top of Page |
EDITOR
Neil Love, MD
INTERVIEWS
Paul A Bunn Jr, MD
- Select publications
Nasser H Hanna, MD
- Select publications
Antoinette J Wozniak, MD
- Select publications
A CME Audio Series and Activity