
| Tracks 1-14 |
| Track 1 |
EORTC study of prophylactic cranial irradiation (PCI) versus no PCI in extensive-disease small cell lung cancer (SCLC) after response to chemotherapy |
| Track 2 |
Clinical implications of the EORTC PCI trial results |
| Track 3 |
Neurologic and cognitive side effects of cranial irradiation |
| Track 4 |
Mortality and disability from brain metastases in extensive-disease SCLC |
| Track 5 |
Integration of bevacizumab with chemoradiation therapy (chemoRT) for patients with solid tumors |
| Track 6 |
Potential risks and benefits of chemoRT with bevacizumab in lung cancer |
| Track 7 |
ECOG-E1505 evaluating adjuvant chemotherapy with or without bevacizumab in Stage IB to IIIA non-small cell lung cancer (NSCLC) |
|
| Track 8 |
Role of adjuvant chemotherapy for patients with Stage IB NSCLC |
| Track 9 |
Clinical and molecular predictors
of response to erlotinib |
| Track 10 |
Quality of life with erlotinib versus chemotherapy |
| Track 11 |
Implications of the HOG LUN 01-24 trial results: Docetaxel consolidation in Stage III NSCLC |
| Track 12 |
Risk of relapse after chemoRT in
Stage III NSCLC |
| Track 13 |
SWOG-S0023: Maintenance
gefitinib or placebo after
chemoRT/docetaxel for patients
with Stage III NSCLC |
| Track 14 |
Proposed RTOG study of
higher-dose radiation therapy
in Stage III NSCLC |
|
|
Select Excerpts from the Interview
Tracks 1-2
 DR LOVE:
DR LOVE: What is your take on the plenary presentation at ASCO on the
EORTC study of PCI for extensive-disease SCLC (Slotman 2007)?
 DR CURRAN: PCI is a standard approach for patients with limited-stage
SCLC who achieve a complete or near-complete response to chest radiation
therapy and chemotherapy. At least two meta-analyses demonstrated an overall
absolute increase in survival of five percent with that approach over a series of studies and, in general, approximately a 50 percent or greater reduction in the
development of central nervous system metastases (Aupérin 1999; Fried 2004).
DR CURRAN: PCI is a standard approach for patients with limited-stage
SCLC who achieve a complete or near-complete response to chest radiation
therapy and chemotherapy. At least two meta-analyses demonstrated an overall
absolute increase in survival of five percent with that approach over a series of studies and, in general, approximately a 50 percent or greater reduction in the
development of central nervous system metastases (Aupérin 1999; Fried 2004).
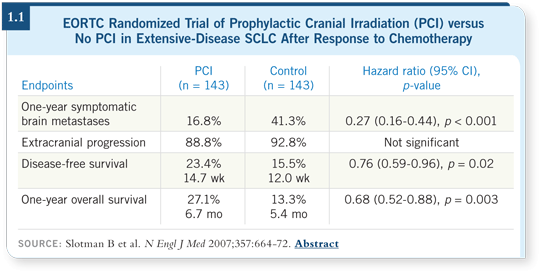
The decision was made by the EORTC to conduct a trial to determine
whether PCI could confer the same level of benefit to patients with extensive-stage
disease who had a response to chemotherapy (Slotman 2007; [1.1]). The
random assignment of 286 patients was between the administration of PCI at
an aggressive fractionation of 20 Gray in five 4-Gray fractions versus observation.
An important factor was that no brain imaging was required to confirm
eligibility, so it’s possible that patients already had asymptomatic metastases
at the time of randomization. One interpretation of the study was: could this
PCI have been early treatment of subclinical disease?
A statistically significant improvement was seen, not only in progression-free
survival but also overall survival, which was a startling observation considering
the fact that patients with extensive-stage SCLC, even those who experienced
a good response to chemotherapy, have so many competing risks for mortality.
One can interpret these data in several ways: (1) It was a positive study, which
defines a new paradigm of treatment for extensive-stage SCLC, (2) It was a
positive study that is so counterintuitive that it needs confirmation or (3) It
was a positive study that has to do with design methodology — for example,
lack of careful restaging of the patients to assess response or evaluate the CNS
and perhaps asymmetry in the actual randomization and stratification.
 DR LOVE: How were you approaching these patients before the EORTC
study was presented, and what are you doing now?
DR LOVE: How were you approaching these patients before the EORTC
study was presented, and what are you doing now?
 DR CURRAN: These patients have extensive-stage disease — that’s an old
VA Lung Cancer Group definition based on being encompassable or not in a
reasonable radiation field. Some have extensive-stage disease and what I call
oligometastases with an excellent complete response or near-complete response
to chemotherapy or chemoradiation therapy, with whom I have a discussion
about an aggressive therapeutic approach, including PCI. However, this
is dependent on them having a normal brain MRI after staging. I have been doing that for highly selected patients, and I will continue to. As far as the
broader group of patients with extensive-stage SCLC, I have not changed
my practice.
DR CURRAN: These patients have extensive-stage disease — that’s an old
VA Lung Cancer Group definition based on being encompassable or not in a
reasonable radiation field. Some have extensive-stage disease and what I call
oligometastases with an excellent complete response or near-complete response
to chemotherapy or chemoradiation therapy, with whom I have a discussion
about an aggressive therapeutic approach, including PCI. However, this
is dependent on them having a normal brain MRI after staging. I have been doing that for highly selected patients, and I will continue to. As far as the
broader group of patients with extensive-stage SCLC, I have not changed
my practice.
 DR LOVE: What was the overall consensus within the RTOG regarding the
EORTC study results?
DR LOVE: What was the overall consensus within the RTOG regarding the
EORTC study results?
 DR CURRAN: The responses span the spectrum. Some members say it has
changed how they approach these patients, whereas others found the design so
different from our standards that they don’t know how to apply the data.
DR CURRAN: The responses span the spectrum. Some members say it has
changed how they approach these patients, whereas others found the design so
different from our standards that they don’t know how to apply the data.
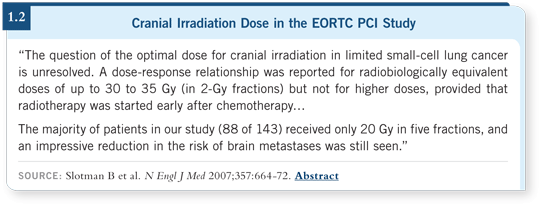
I have never used the specific radiation regimen from the EORTC study, nor
has any American study ever used it for PCI. This was administered in a week
in five 4-Gray fractions (1.2), and the only time I see that in the US is for
patients with cerebral metastases who are in poor condition, for whom there’s
a desire to complete treatment rapidly. For PCI, the usual treatment I administer
is 2.5 Gray in 10 fractions.
Tracks 5-6
 DR LOVE:
DR LOVE: What do you think about the clinical research strategy of
combining radiation therapy with bevacizumab?
 DR CURRAN: The landmark paper by Chris Willett and Rakesh Jain showed
that in a small number of rectal cancer patients, bevacizumab alone had a
physiologic effect in terms of the vasculature and showed clinical effect when
combined with radiation therapy (Willett 2004).
DR CURRAN: The landmark paper by Chris Willett and Rakesh Jain showed
that in a small number of rectal cancer patients, bevacizumab alone had a
physiologic effect in terms of the vasculature and showed clinical effect when
combined with radiation therapy (Willett 2004).
 DR LOVE: The other major discovery from that study was not only is there an
anti-angiogenic effect, but there is also a normalization of the tumor vasculature,
which has big implications in chemotherapy and radiation therapy.
DR LOVE: The other major discovery from that study was not only is there an
anti-angiogenic effect, but there is also a normalization of the tumor vasculature,
which has big implications in chemotherapy and radiation therapy.
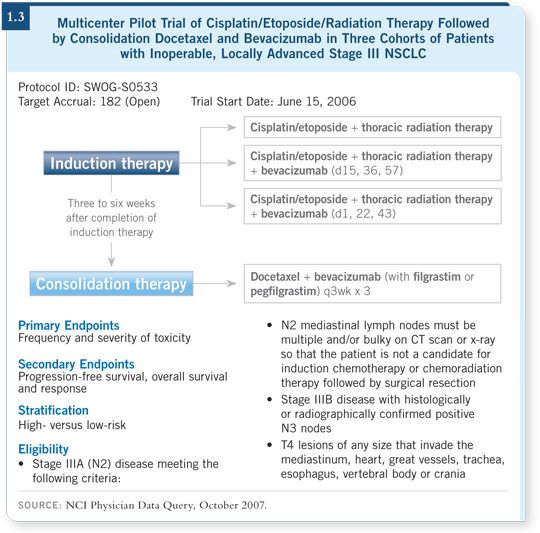
 DR CURRAN: Yes, because tumor hypoxia is thought to be one of the primary
mechanisms of resistance to radiation therapy — you’re right. SWOG initiated
a study in Stage III NSCLC that is integrating bevacizumab into chemoradiation
therapy, dividing patients between high- and low-risk groups according
to whether they have central tumors, squamous histology, history of hemoptysis
and other factors (SWOG-S0533; [1.3]). Due to cautious enrollment, a small number of patients have enrolled. Episodes of tracheoesophageal (TE)
fistula were reported in parallel ongoing studies in SCLC with bevacizumab
and chemoradiation therapy. That required SWOG to stop S0533 and evaluate
safety.
DR CURRAN: Yes, because tumor hypoxia is thought to be one of the primary
mechanisms of resistance to radiation therapy — you’re right. SWOG initiated
a study in Stage III NSCLC that is integrating bevacizumab into chemoradiation
therapy, dividing patients between high- and low-risk groups according
to whether they have central tumors, squamous histology, history of hemoptysis
and other factors (SWOG-S0533; [1.3]). Due to cautious enrollment, a small number of patients have enrolled. Episodes of tracheoesophageal (TE)
fistula were reported in parallel ongoing studies in SCLC with bevacizumab
and chemoradiation therapy. That required SWOG to stop S0533 and evaluate
safety.
 DR LOVE: What’s your perspective on the potential benefits and risks of
bevacizumab combined with chemoradiation therapy in lung cancer?
DR LOVE: What’s your perspective on the potential benefits and risks of
bevacizumab combined with chemoradiation therapy in lung cancer?
 DR CURRAN: My gut feeling is that it can be an active addition to chemoradiation
therapy. My biggest concern is that people will become legitimately
concerned about the risk of catastrophic complications, and that will slow
the clinical development. TE fistulas are obviously serious, life-threatening
events. Originally with thoracic malignancies, we saw these when we started
combining chemotherapy with radiation therapy decades ago, and it made
some people back away from that paradigm. However, once we learned how
to administer it, it revolutionized the care for those patients.
DR CURRAN: My gut feeling is that it can be an active addition to chemoradiation
therapy. My biggest concern is that people will become legitimately
concerned about the risk of catastrophic complications, and that will slow
the clinical development. TE fistulas are obviously serious, life-threatening
events. Originally with thoracic malignancies, we saw these when we started
combining chemotherapy with radiation therapy decades ago, and it made
some people back away from that paradigm. However, once we learned how
to administer it, it revolutionized the care for those patients.
Even further back in the radiation therapy-alone era of unresected thoracic
malignancies, we were taught that if someone had a tumor potentially invading the esophagus, we were to use a lower dose per fraction to avoid
a TE fistula. So I view the TE fistula as a surrogate for excellent tumor
response. We just need to figure out how to calibrate the antitumor action so
it doesn’t have a catastrophic effect.
Tracks 9-10
 DR LOVE:
DR LOVE: Can you discuss what we know about predictors of response to
EGFR tyrosine kinase inhibitors (TKIs), specifically erlotinib?
 DR CURRAN: There are molecular and epidemiologic predictors of response
to EGFR TKIs in second- and third-line treatment of NSCLC. Patients who
are never smokers, women and of Asian descent have a higher likelihood of
responding to a TKI than men who are heavy smokers and non-Asian.
DR CURRAN: There are molecular and epidemiologic predictors of response
to EGFR TKIs in second- and third-line treatment of NSCLC. Patients who
are never smokers, women and of Asian descent have a higher likelihood of
responding to a TKI than men who are heavy smokers and non-Asian.
In searching for predictors of response at the molecular level, the focus is
on mutations in chromosomes 18 and 22. Work is also being conducted at
Harvard, Sloan-Kettering and Colorado showing that EGFR mutational
analysis is extremely helpful, in the right hands. The FISH-type analysis by
Fred Hirsch and others has also been useful for predicting response (Cappuzzo
2005a, 2005b; Hirsch 2003, 2005, 2007).
Recently, we’ve seen interest in whether the presence of a K-ras mutation
is a sufficiently adverse predictor of response to warrant not using TKIs.
I reviewed data suggesting that, although the overall response rate using
RECIST is lower in patients with K-ras mutation-positive disease, waterfall-type
trends clearly suggest that the range of responses does not appear different
in those patients with K-ras mutations from those with non-K-ras mutations.
 DR LOVE: What do you see in terms of quality of life with chemotherapy
versus erlotinib?
DR LOVE: What do you see in terms of quality of life with chemotherapy
versus erlotinib?
 DR CURRAN: In general, erlotinib is better tolerated, especially compared
to doublet-based chemotherapy. If erlotinib provides the same palliation and
arrest of symptoms as doublet chemotherapy in the older, never smoker or
oligosmoker with a poor performance status at diagnosis, I would like to have
data to support erlotinib as initial treatment for that patient. That’s an option
that many patients and families would prefer.
DR CURRAN: In general, erlotinib is better tolerated, especially compared
to doublet-based chemotherapy. If erlotinib provides the same palliation and
arrest of symptoms as doublet chemotherapy in the older, never smoker or
oligosmoker with a poor performance status at diagnosis, I would like to have
data to support erlotinib as initial treatment for that patient. That’s an option
that many patients and families would prefer.
Select Publications

