
| Tracks 1-17 |
| Track 1 |
Introduction |
| Track 2 |
Adjuvant chemotherapy in Stage IB non-small cell lung cancer (NSCLC): Update of CALGB-9633 |
| Track 3 |
Clinical implications of CALGB-9633 and the treatment of Stage IB disease |
| Track 4 |
Lung Adjuvant Cisplatin Evaluation (LACE): A pooled analysis of five randomized clinical trials including 4,584 patients |
| Track 5 |
Adjuvant chemotherapy in elderly patients: Analysis of CAN-NCIC-BR10 |
| Track 6 |
Side effects of bevacizumab and eligibility criteria for adjuvant trials |
| Track 7 |
ECOG-E1505: Adjuvant chemotherapy with or without bevacizumab in patients with completely resected Stage IB-IIIA NSCLC |
| Track 8 |
Incorporation of bevacizumab into the treatment of advanced NSCLC |
| Track 9 |
Phase II trial of docetaxel with the multikinase inhibitor ZD6474 as second-line therapy |
|
| Track 10 |
Use of bevacizumab in patients with treated brain metastases |
| Track 11 |
Potential relationship between hemorrhage and response to bevacizumab |
| Track 12 |
Multicenter, randomized trial of bevacizumab with chemotherapy (docetaxel or pemetrexed) or erlotinib compared to chemotherapy alone for recurrent or refractory NSCLC |
| Track 13 |
Predictors of response to the EGFR tyrosine kinase inhibitors (TKIs) |
| Track 14 |
Use of erlotinib in nonsmokers with early or locally advanced NSCLC |
| Track 15 |
Hoosier Oncology Group trial: Cisplatin/etoposide/radiation therapy with or without consolidation docetaxel in advanced Stage III NSCLC |
| Track 16 |
Potential role for nanoparticle albumin-bound (nab) paclitaxel in NSCLC |
| Track 17 |
Decreasing morbidity in patients with locally advanced disease undergoing combined-modality therapy |
|
|
Select Excerpts from the Interview
Tracks 2-3
 DR LOVE:
DR LOVE: Can you describe the study design of CALGB-9633 (Strauss
2006) and discuss the outcomes?
 DR LANGER: The CALGB-9633 study was an adjuvant trial of paclitaxel/carboplatin versus observation for patients with Stage IB disease. The trial
specifically evaluated patients without nodal involvement in tumors that
were at least three centimeters or when there was invasion of the main-stem
bronchus through the visceral pleura.
DR LANGER: The CALGB-9633 study was an adjuvant trial of paclitaxel/carboplatin versus observation for patients with Stage IB disease. The trial
specifically evaluated patients without nodal involvement in tumors that
were at least three centimeters or when there was invasion of the main-stem
bronchus through the visceral pleura.
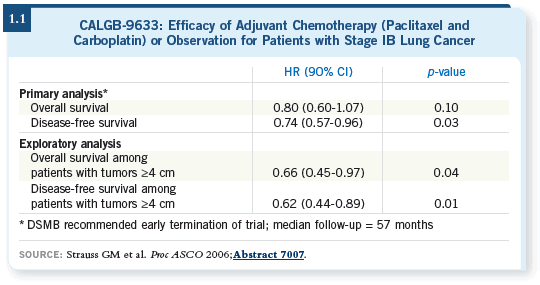
The initial report from two years ago (Strauss 2004) showed a statistically
significant survival improvement in patients who received chemotherapy.
About a 12 percent improvement was seen in four-year survival. In the control
arm, the four-year overall survival was 59 percent. For the intervention arm it
was 71 percent (p = 0.028). The majority of patients were actually able to get
all four cycles at or near full dose.
The update at the 2006 ASCO meeting (Strauss 2006) was tremendously
disappointing — the p-value increased to 0.1. The five-year absolute survival
difference was two to three percent (1.1).
 DR LOVE: How do you interpret those findings?
DR LOVE: How do you interpret those findings?
 DR LANGER: Because the investigators had not observed the predetermined
number of events, this may be a premature reporting of a Phase III trial.
DR LANGER: Because the investigators had not observed the predetermined
number of events, this may be a premature reporting of a Phase III trial.
Another interesting caveat is that, according to a retrospective analysis,
patients whose tumors were four centimeters or larger actually had a survival
benefit. Those results have left us in a therapeutic quandary. What do we do
with Stage IB patients? Do we treat them? Do we observe them? Do we replicate
this regimen in this group of patients? Do we segregate them by tumor
size, using four centimeters as our cutoff ? And if we do treat them, do we use
paclitaxel and carboplatin or a cisplatin-containing regimen?
People have evaluated the data and dismissed the role of carboplatin. Frankly,
I don’t believe we have sufficient data. Only one trial evaluated carboplatin in
the adjuvant treatment of patients with Stage IB disease only. Carboplatin was
not evaluated in patients with Stage II or Stage IIIA disease.
I must confess that since ASCO, at least for patients with Stage II or IIIA disease,
I’ve started using cisplatin. For patients with tumors that are four centimeters or
larger in size with Stage IB disease, I’m still using paclitaxel and carboplatin.
Track 4
 DR LOVE:
DR LOVE: Can you discuss the data from the meta-analysis of trials
evaluating adjuvant cisplatin?
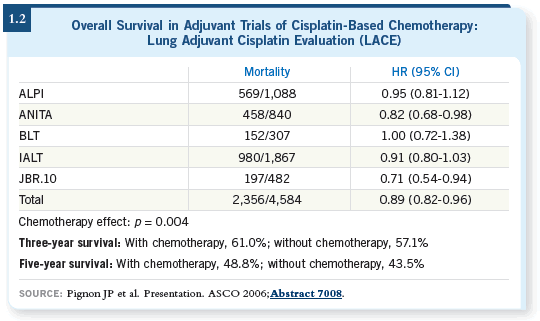
 DR LANGER: The Lung Adjuvant Cisplatin Evaluation (Pignon 2006) evaluated
three positive platinum trials — ANITA, JBR.10 and the IAL trial
— and two other trials: the ALPI trial from Italy and the BLT trial, primarily
from the United Kingdom — both of which were negative. So in fairness,
it evaluated both positive and negative trials, and in aggregate, despite the
inclusion of the negative trials, a robust, statistically significant improvement
in survival with a platinum-based regimen was seen — overall about a five
percent difference at five years (1.2).
DR LANGER: The Lung Adjuvant Cisplatin Evaluation (Pignon 2006) evaluated
three positive platinum trials — ANITA, JBR.10 and the IAL trial
— and two other trials: the ALPI trial from Italy and the BLT trial, primarily
from the United Kingdom — both of which were negative. So in fairness,
it evaluated both positive and negative trials, and in aggregate, despite the
inclusion of the negative trials, a robust, statistically significant improvement
in survival with a platinum-based regimen was seen — overall about a five
percent difference at five years (1.2).
In their further analysis, that benefit was essentially confined to patients with
Stage II or IIIA disease. When the investigators evaluated Stage IA disease,
chemotherapy seemed to be associated with a detrimental outcome, and the
effect in Stage IB disease wasn’t significant. It was trending in the right direction,
but the p-value wasn’t significant and the confidence intervals clearly
overlapped.
Track 5
 DR LOVE:
DR LOVE: Can you discuss the analysis of the Canadian JBR.10 study that
reported data on adjuvant treatment in the elderly (Pepe 2006)?
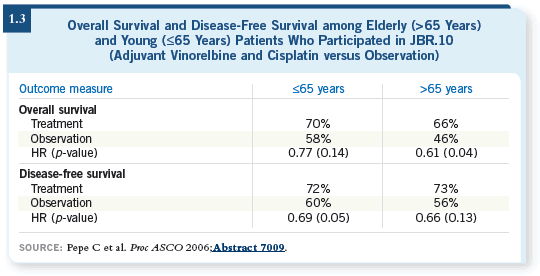
 DR LANGER: It was one of the first analyses of the elderly in the adjuvant
setting. The JBR.10 trial subanalysis of elderly patients was presented by
Carmella Pepe at the 2006 ASCO meeting during the lung plenary session.
They used 65 years of age as their cutoff.
DR LANGER: It was one of the first analyses of the elderly in the adjuvant
setting. The JBR.10 trial subanalysis of elderly patients was presented by
Carmella Pepe at the 2006 ASCO meeting during the lung plenary session.
They used 65 years of age as their cutoff.
Patients who were older than 65 years of age constituted a third of the total
accrual to JBR.10, so it was a fairly large group. Among the elderly, a higher
percentage of patients had squamous histology, which is no surprise because it’s
probably a result of the use of cigarettes.
The upshot was that the elderly had a significant survival benefit. The younger
patients did better across the board, but relatively speaking, comparing chemotherapy
to observation, there was still a survival benefit among the elderly (1.3).
Select Publications

