
| Tracks 1-13 |
| Track 1 |
Introduction |
| Track 2 |
Background to ECOG trial E4599:
Carboplatin/paclitaxel with or
without bevacizumab |
| Track 3 |
ECOG-E4599: Side effects and
toxicity |
| Track 4 |
Factors associated with
pulmonary hemorrhage in
patients with lung cancer treated
with bevacizumab |
| Track 5 |
ECOG-E4599 subset analysis of
survival by gender |
| Track 6 |
Prospective correlative
assessment of biomarkers in
E4599 |
| Track 7 |
Updated analysis of patient
deaths in E4599 |
|
| Track 8 |
Clinical implications of E4599 |
| Track 9 |
NCT00095225: Bevacizumab
with chemotherapy (docetaxel
or pemetrexed) or erlotinib
compared to chemotherapy alone
for recurrent or refractory non-small
cell lung cancer (NSCLC) |
| Track 10 |
Potential role for bevacizumab/
erlotinib without chemotherapy |
| Track 11 |
ECOG-E1505 adjuvant study of
chemotherapy with or without
bevacizumab |
| Track 12 |
Development of the EGFR and
VEGF tyrosine kinase inhibitor
ZD6474 (vandetanib) |
| Track 13 |
Nanoparticle paclitaxel in the
treatment of lung cancer |
|
|
Select Excerpts from the Interview
Track 8
 DR LOVE:
DR LOVE: When you presented the data from ECOG-E4599 in 2005 (Sandler 2005), one of your concluding comments was that first-line carboplatin, paclitaxel and bevacizumab is a new standard of care for NSCLC patients who would have been eligible for the study. Do you still feel that way?
 DR SANDLER: Yes. My specific quote was that this combination in the nonsquamous-cell population included in ECOG-E4599 was the new ECOG reference standard. I certainly would acknowledge that some toxicity associated
with the regimen appears greater than with chemotherapy alone, but the benefits that were seen with respect to all the outcomes, including survival, outweigh the risks.
DR SANDLER: Yes. My specific quote was that this combination in the nonsquamous-cell population included in ECOG-E4599 was the new ECOG reference standard. I certainly would acknowledge that some toxicity associated
with the regimen appears greater than with chemotherapy alone, but the benefits that were seen with respect to all the outcomes, including survival, outweigh the risks.
We did not specifically look at quality of life in the study, but it appears that other nonspecific toxicities, such as fatigue, were not increased and that quality of life in fact improved, because in virtually every other study that has shown an improvement in survival, quality of life has been better.
 DR LOVE: The trial stipulated that bevacizumab would be continued alone after the chemotherapy until the disease progresses. Do you do that in your practice?
DR LOVE: The trial stipulated that bevacizumab would be continued alone after the chemotherapy until the disease progresses. Do you do that in your practice?
 DR SANDLER: Yes, that is exactly what I do. I follow the intent and guidelines of ECOG-E4599. The potential benefit of the maintenance portion of the bevacizumab has been widely recognized.
I no longer have any patients actively being treated on ECOG-E4599. I do have one patient who went between a year and a half and a year and three quarters and was on a maintenance dose of bevacizumab, and I have some other patients who had gone not quite that far.
DR SANDLER: Yes, that is exactly what I do. I follow the intent and guidelines of ECOG-E4599. The potential benefit of the maintenance portion of the bevacizumab has been widely recognized.
I no longer have any patients actively being treated on ECOG-E4599. I do have one patient who went between a year and a half and a year and three quarters and was on a maintenance dose of bevacizumab, and I have some other patients who had gone not quite that far.
Track 4
 DR LOVE:
DR LOVE: Can you discuss the data that you presented at ASCO 2006 on pulmonary hemorrhage in patients treated with bevacizumab in the study?
 DR SANDLER: We submitted a poster that attempted to define prognostic variables for pulmonary hemorrhage (Sandler 2006). It was a case control study in which we combined the data sets from a Phase II study with those from the ECOG-E4599 study and attempted to assess a wide range of prognostic variables to see if one could better define which group of patients was more at risk.
DR SANDLER: We submitted a poster that attempted to define prognostic variables for pulmonary hemorrhage (Sandler 2006). It was a case control study in which we combined the data sets from a Phase II study with those from the ECOG-E4599 study and attempted to assess a wide range of prognostic variables to see if one could better define which group of patients was more at risk.
We looked at 22 patients with Grade III or higher pulmonary hemorrhage. Not surprising with the limited number of patients, nothing was statistically significant, but there appeared to be trends for patients with baseline cavitation in their tumors and a history of hemoptysis that predated treatment.
 DR LOVE:
DR LOVE: Hemoptysis wasn’t allowed in the study, correct?
 DR SANDLER: Correct. In ECOG-E4599, it was not specifically written into the study at first, but then one or more patients entered the study who had hemoptysis. After the first 60 or so patients, it was put in specifically as an exclusion criterion.
DR SANDLER: Correct. In ECOG-E4599, it was not specifically written into the study at first, but then one or more patients entered the study who had hemoptysis. After the first 60 or so patients, it was put in specifically as an exclusion criterion.
 DR LOVE:
DR LOVE: Oncologists are interested in whether location — peripheral versus proximal — was a factor, and I would also be interested in whether a correlation
appeared with tumor bulk.
 DR SANDLER: We observed size using three centimeters as a cutoff, and it did not seem to correlate. We noted location, but when radiologists look at what they define as central tumors, it really encompasses a wide range of tumors because I believe their definition is anything that’s more than two centimeters away from the pleural reflection.
DR SANDLER: We observed size using three centimeters as a cutoff, and it did not seem to correlate. We noted location, but when radiologists look at what they define as central tumors, it really encompasses a wide range of tumors because I believe their definition is anything that’s more than two centimeters away from the pleural reflection.
In our study, we had an independent radiology group examine all the
individual CAT scans — so they were all independently reviewed — and size
and location did not seem to matter. We saw a hint that endobronchial disease
might be an issue, although that was not statistically significant and it is a very
difficult interpretation on a CAT scan, and the results were inconsistent across
all the CAT scans and techniques.
Track 9
 DR LOVE:
DR LOVE: Can you update us on the erlotinib/bevacizumab combination
data that were presented at ASCO?
 DR SANDLER: The combination of erlotinib and bevacizumab involves the
inhibition of the EGFR pathway along with angiogenesis and was based on a
Phase I and II study conducted by Dr Roy Herbst at MD Anderson and my
colleagues and me at Vanderbilt, in which we had 40 patients with previously
treated nonsquamous-cell, non-small cell lung cancer.
DR SANDLER: The combination of erlotinib and bevacizumab involves the
inhibition of the EGFR pathway along with angiogenesis and was based on a
Phase I and II study conducted by Dr Roy Herbst at MD Anderson and my
colleagues and me at Vanderbilt, in which we had 40 patients with previously
treated nonsquamous-cell, non-small cell lung cancer.
We saw a 20 percent response rate, a roughly seven-month progression-free
survival and a 12.5-month median survival (Sandler 2004), but because it was
a Phase I/II limited-institution study, a subsequent larger-scale randomized
Phase II study was conducted that involved 120 patients and was presented at
ASCO (Fehrenbacher 2006).
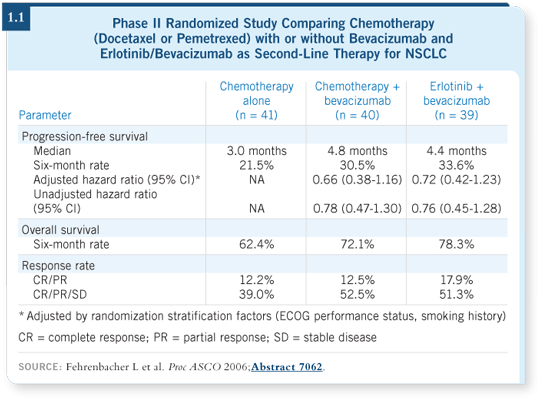
This study had three arms: Bevacizumab in combination with either chemotherapy
(docetaxel or pemetrexed) or erlotinib compared with chemotherapy
alone. An improvement in progression-free survival favored the two bevacizumab
arms. It was three months on the chemotherapy alone arm and roughly
four and a half months on the two bevacizumab arms — 4.4 months with
erlotinib and 4.8 months with chemotherapy (1.1).
Track 10
 DR LOVE:
DR LOVE: Would it be correct to say that you observed the same results
by adding erlotinib to bevacizumab as with adding chemotherapy to
bevacizumab? You would expect fewer side effects with the double
biologic therapy than with chemotherapy and bevacizumab.
 DR SANDLER: That’s an interesting point, and that is my take on this study.
It’s another example that the addition of bevacizumab to chemotherapy or,
in this case, a biologic, an EGFR tyrosine kinase (TK) inhibitor, such as
erlotinib, showed benefit when compared to chemotherapy alone. That’s the
first point.
DR SANDLER: That’s an interesting point, and that is my take on this study.
It’s another example that the addition of bevacizumab to chemotherapy or,
in this case, a biologic, an EGFR tyrosine kinase (TK) inhibitor, such as
erlotinib, showed benefit when compared to chemotherapy alone. That’s the
first point.
The second point is that it now provides evidence that a non-chemotherapy based
approach in the second-line setting consisting of erlotinib and bevacizumab
might prove to be equivalent to chemotherapy.
A large Phase III study of more than 600 patients is evaluating erlotinib alone
versus erlotinib and bevacizumab in the second-line setting (OSI3364g).
 DR LOVE:
DR LOVE: From the practical or clinical perspective of a physician in practice,
what are the implications of these data?
 DR SANDLER: That’s another very good question. We do not have official
randomized Phase III data for the biologic doublet, but we certainly have
enough evidence — more evidence than we have for chemotherapy in the
third-, fourth- and fifth-line settings, which we are all guilty of administering.
DR SANDLER: That’s another very good question. We do not have official
randomized Phase III data for the biologic doublet, but we certainly have
enough evidence — more evidence than we have for chemotherapy in the
third-, fourth- and fifth-line settings, which we are all guilty of administering.
In clinical practice, this combination would be a bit of a reach at this point. It
would be intriguing for those patients who are selected for the use of erlotinib
— nonsmokers, Asian patients, patients with EGFR mutations, FISH-positive
cases, et cetera.
In our Phase I/II study there was a hint that this combination was working in
patients with and without EGFR mutations, smokers and nonsmokers. That
has not been broken out yet for the randomized Phase II study, but it’s possible
that the combination may allow for the use of the EGFR agent in a broader
range of patients.
 DR LOVE:
DR LOVE: What is seen in terms of side effects and toxicity with this biologic
doublet, both in terms of data and your own clinical experience?
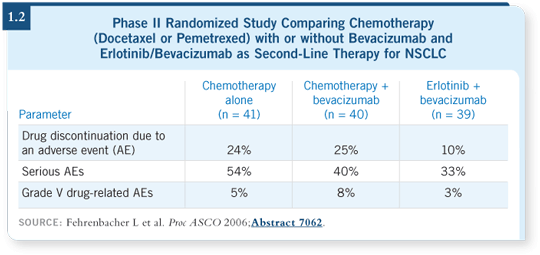
 DR SANDLER: No surprises have occurred in toxicity (1.2). We’re seeing rash,
minimal diarrhea associated with the EGFR TK inhibitor, some proteinuria
and hypertension related to bevacizumab. Three deaths related to pulmonary
hemorrhage occurred on the study — two on the chemotherapy/bevacizumab
arm and one on the erlotinib/bevacizumab arm (Fehrenbacher 2006).
DR SANDLER: No surprises have occurred in toxicity (1.2). We’re seeing rash,
minimal diarrhea associated with the EGFR TK inhibitor, some proteinuria
and hypertension related to bevacizumab. Three deaths related to pulmonary
hemorrhage occurred on the study — two on the chemotherapy/bevacizumab
arm and one on the erlotinib/bevacizumab arm (Fehrenbacher 2006).
Track 12
 DR LOVE:
DR LOVE: I want to get your take on a couple of new developments in
lung cancer clinical research. What are your thoughts about ZD6474, or
vandetanib?
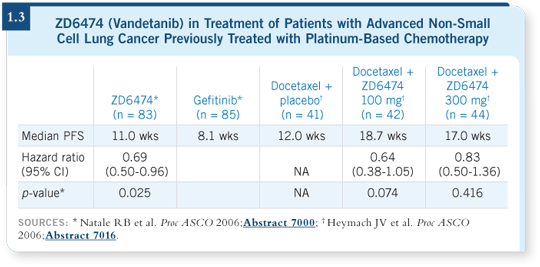
 DR SANDLER: ZD6474 is an oral agent that has both EGFR and VEGF TK
inhibition, and it has been involved in two randomized Phase II studies (1.3).
DR SANDLER: ZD6474 is an oral agent that has both EGFR and VEGF TK
inhibition, and it has been involved in two randomized Phase II studies (1.3).
Ron Natale presented a study of ZD6474 compared to gefitinib and demonstrated
an improvement in time to progression with ZD6474 (Natale 2006).
John Heymach from MD Anderson presented another study evaluating
docetaxel with or without ZD6474, which also showed an improvement in
time to progression with the combination, favoring a lower dose in that setting
(Heymach 2006).
 DR LOVE:
DR LOVE: Is ZD6474 sort of the oral tyrosine kinase inhibitor version of your
doublet of erlotinib and bevacizumab?
 DR SANDLER: Correct, that’s the theory.
DR SANDLER: Correct, that’s the theory.
The interesting aspect is that apparently, depending on the dose, you may see
one effect over the other. At 100 mg, the effect appears to be more of an anti-angiogenic
one, and that may explain why in combination with chemotherapy
the lower dose seems to be better, but the higher dose when used on its own,
which has both effects, seems to be better than the lower dose.
 DR LOVE:
DR LOVE: Where do you think we will be heading with this agent? Will we
be using it in clinical practice in the near future, and if so, where and how?
 DR SANDLER: Randomized Phase III studies are now going forward. If the
results are positive, it will become a player in the treatment of metastatic non-small
cell lung cancer.
DR SANDLER: Randomized Phase III studies are now going forward. If the
results are positive, it will become a player in the treatment of metastatic non-small
cell lung cancer.
Select publications

