You are here: Home: LCU 1 | 2006 : Richard J Gralla, MD

| Tracks 1-17 |
| Track 1 |
Introduction by Neil Love, MD |
| Track 2 |
Advances in chemotherapy for
metastatic NSCLC |
| Track 3 |
Comparison of platinum agents in
the treatment of NSCLC |
| Track 4 |
Choice of chemotherapy in
combination with cisplatin or
carboplatin |
| Track 5 |
ECOG-E4599: Carboplatin/
paclitaxel with or without
bevacizumab in advanced, non-squamous
cell NSCLC |
| Track 6 |
Bevacizumab-associated side
effects in ECOG-E4599 |
| Track 7 |
Clinical implications of ECOG-E4599 |
| Track 8 |
EGFR mutation as a predictor of
response to TKIs |
| Track 9 |
Nonsmoking as a predictor of
response to TKIs |
| Track 10 |
Selection of patients for treatment
with TKIs with or without
chemotherapy |
|
| Track 11 |
Pooled analysis of 12 studies:
Survival and magnitude of benefit
from adjuvant chemotherapy |
| Track 12 |
Selection of the optimal adjuvant
chemotherapeutic regimen |
| Track 13 |
Recent neoadjuvant clinical trial
results in patients with Stage III
disease |
| Track 14 |
SWOG-S0023: Concurrent
chemoradiotherapy followed
by consolidation docetaxel and
gefitinib/placebo maintenance in
unresectable Stage III NSCLC |
| Track 15 |
Topoisomerase inhibitors and
cisplatin in the treatment of
extensive SCLC |
| Track 16 |
Imatinib in patients with c-kit-expressing
relapsed SCLC |
| Track 17 |
Adjuvant versus neoadjuvant
therapy in NSCLC |
| |
|
| |
|
|
|
Select Excerpts from the Interview
 Track 3 Track 3
 DR LOVE: What’s your take on the issue of cisplatin versus carboplatin for
metastatic disease? DR LOVE: What’s your take on the issue of cisplatin versus carboplatin for
metastatic disease? |
 DR GRALLA: At the ASCO 2005 meeting, a meta-analysis that demonstrated
two-drug combinations containing a platinum agent are better than nonplatinum-containing regimens also showed the cisplatin-based combinations to be
more effective (Barlesi 2005). This analysis showed approximately a 12 percent survival advantage among patients treated with a cisplatin-based regimen. A
bit more toxicity occurred with the cisplatin-containing combinations, but the
toxicity was classified as acceptable. DR GRALLA: At the ASCO 2005 meeting, a meta-analysis that demonstrated
two-drug combinations containing a platinum agent are better than nonplatinum-containing regimens also showed the cisplatin-based combinations to be
more effective (Barlesi 2005). This analysis showed approximately a 12 percent survival advantage among patients treated with a cisplatin-based regimen. A
bit more toxicity occurred with the cisplatin-containing combinations, but the
toxicity was classified as acceptable.
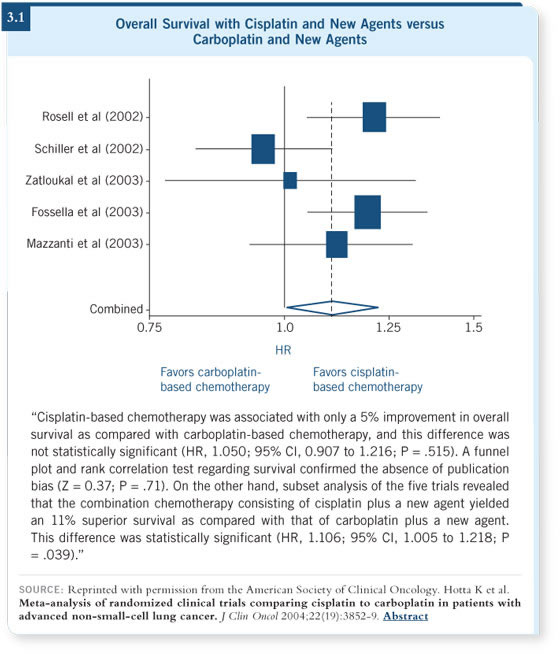
In the past year, two other meta-analyses have examined cisplatin versus
carboplatin with newer agents, including a Japanese meta-analysis published by
Hotta (Hotta 2005; [3.1]).
They both showed that adding the same newer agent to both platinums
resulted in a 12 to 15 percent survival advantage for cisplatin. So survival
appears to be a bit better with the platinum combinations versus nonplatinum,
and while nobody particularly likes cisplatin, it appears to be superior to
carboplatin.
 Track 5 Track 5
 DR LOVE: What were your thoughts on the ECOG-E4599 study with
bevacizumab presented by Alan Sandler (Sandler 2005)? DR LOVE: What were your thoughts on the ECOG-E4599 study with
bevacizumab presented by Alan Sandler (Sandler 2005)? |
 DR GRALLA: The big winner at ASCO 2005 was bevacizumab. The ECOG-E4599
trial was certainly an interesting study. It consisted of nearly 900
patients with nonsquamous cell lung cancers, and all the patients received
paclitaxel/carboplatin. They were then randomly assigned to receive bevacizumab
at 15 mg/kg every three weeks or not. DR GRALLA: The big winner at ASCO 2005 was bevacizumab. The ECOG-E4599
trial was certainly an interesting study. It consisted of nearly 900
patients with nonsquamous cell lung cancers, and all the patients received
paclitaxel/carboplatin. They were then randomly assigned to receive bevacizumab
at 15 mg/kg every three weeks or not.
The data demonstrated approximately a 10.2-month survival with paclitaxel/
carboplatin, which is probably the best rate we have seen reported for
that combination, and a 12.5-month survival with the bevacizumab, which
indicates a 15 to 18 percent benefit to adding bevacizumab.
 DR LOVE: What are the clinical implications of these data? DR LOVE: What are the clinical implications of these data?
 DR GRALLA: The difference in efficacy between the two arms was highly
significant. Actually, both arms of the study did better than expected, so it’s
not as though the bevacizumab arm did well because the chemotherapy-only
arm did poorly. DR GRALLA: The difference in efficacy between the two arms was highly
significant. Actually, both arms of the study did better than expected, so it’s
not as though the bevacizumab arm did well because the chemotherapy-only
arm did poorly.
I think this is likely to be a true finding, and I expect a lot of patients will
receive bevacizumab as a result.
This is at least the seventh large trial, each with a minimum of 600 patients,
that has examined a standard chemotherapy regimen combined with a molecularly
targeted agent. All of them have been negative with the exception of
the bevacizumab trial, so I think the chances of this being correct are
relatively high.
 DR LOVE: In the clinical setting, what agent would you combine
with bevacizumab? DR LOVE: In the clinical setting, what agent would you combine
with bevacizumab?
 DR GRALLA: We have seen an advantage reported with bevacizumab and a
variety of chemotherapies in several other malignancies. Its efficacy appears
to be more tumor related than chemotherapy related; obviously, a second trial
using different drugs from those used in E4599 would answer that question,
but my prediction is that its effectiveness is not chemotherapy specific. DR GRALLA: We have seen an advantage reported with bevacizumab and a
variety of chemotherapies in several other malignancies. Its efficacy appears
to be more tumor related than chemotherapy related; obviously, a second trial
using different drugs from those used in E4599 would answer that question,
but my prediction is that its effectiveness is not chemotherapy specific.
 Track 10 Track 10
 DR LOVE: In your practice, how do you treat nonsmoking female patients
with metastatic adenocarcinoma? DR LOVE: In your practice, how do you treat nonsmoking female patients
with metastatic adenocarcinoma? |
 DR GRALLA: Any nonsmoking woman with adenocarcinoma has a high
likelihood of doing well with a TKI. I have treated many of these patients
with a TKI as monotherapy, and within three to four weeks I expect to see
symptomatic relief and some hint of a response on a simple imaging study like
a chest x-ray. DR GRALLA: Any nonsmoking woman with adenocarcinoma has a high
likelihood of doing well with a TKI. I have treated many of these patients
with a TKI as monotherapy, and within three to four weeks I expect to see
symptomatic relief and some hint of a response on a simple imaging study like
a chest x-ray.
We don’t know whether it’s better to administer a TKI or chemotherapy
first, and we need to conduct research to answer that question. As for using
chemotherapy with erlotinib, the results of the two trials evaluating that
weren’t so good.
 DR LOVE: What new agents or combinations are being evaluated for treatment
of recurrent non-small cell lung cancer? DR LOVE: What new agents or combinations are being evaluated for treatment
of recurrent non-small cell lung cancer?
 DR GRALLA: Many second-line agents have been studied, but docetaxel
remains the agent that is used as the comparator and nothing has beaten it to
date, although there are other candidates. It also has shown good evidence in
the first line and is one of our many reasonable choices in that setting. DR GRALLA: Many second-line agents have been studied, but docetaxel
remains the agent that is used as the comparator and nothing has beaten it to
date, although there are other candidates. It also has shown good evidence in
the first line and is one of our many reasonable choices in that setting.
An article in the Journal of Clinical Oncology indicated that the combination of
bevacizumab and erlotinib can be used but we need to identify the specific
patient population for this combination (Herbst 2005).
I don’t believe we can have great confidence that we have found the target
population for bevacizumab, whereas with erlotinib or gefitinib, maybe we
have.
 Track 11 Track 11
 DR LOVE: Would you discuss the use of adjuvant chemotherapy in the
treatment of non-small cell lung cancer? DR LOVE: Would you discuss the use of adjuvant chemotherapy in the
treatment of non-small cell lung cancer? |
 DR GRALLA: The most recent study was reported at the 2005 ASCO meeting.
The ANITA trial was a large, well-executed international trial, with more
than 800 patients who were randomly assigned to postoperative vinorelbine
and cisplatin or no chemotherapy (Douillard 2005). DR GRALLA: The most recent study was reported at the 2005 ASCO meeting.
The ANITA trial was a large, well-executed international trial, with more
than 800 patients who were randomly assigned to postoperative vinorelbine
and cisplatin or no chemotherapy (Douillard 2005).
The distribution of Stage I, II and III disease was almost equal, with slightly
fewer patients having Stage II disease. This study demonstrated, as have others,
a significant advantage to receiving adjuvant chemotherapy.
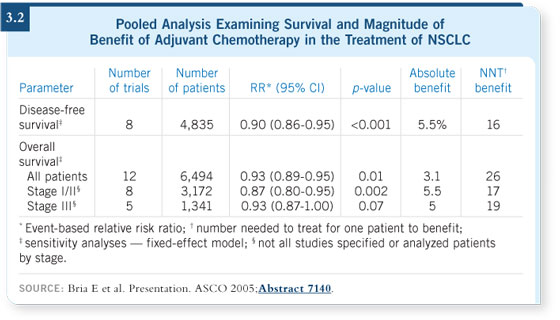
At the ASCO meeting in 2005, Emilio Bria presented a pooled analysis of 11
randomized trials and one meta-analysis with 6,494 patients (Bria 2005; [3.2]).
No matter how the data were segregated — all studies, only those studies
published in peer-review journals, earlier versus later stage — the data showed
a significant advantage with adjuvant chemotherapy.
So I don’t think the question is whether adjuvant chemotherapy improves
survival but rather, what is the magnitude of benefit? Dr Bria’s analysis
provided evidence of an absolute benefit in the three to four percent range,
which is not very much. In breast cancer, the meta-analysis of adjuvant
chemotherapy showed a six percent absolute benefit.
 DR LOVE: What’s the relative risk reduction in lung cancer? DR LOVE: What’s the relative risk reduction in lung cancer?
 DR GRALLA: Interestingly, the smaller the study, the larger the benefit and
vice versa. In individual trials, relative risk benefits or hazard ratios reduced to
0.69 or 0.74. In the ANITA study, the number was 0.79 (Douillard 2005). In the largest study of all, the IALT study, it was 0.86 (Arriagada 2004). When
you look at them all together, the hazard ratio is about 0.9. DR GRALLA: Interestingly, the smaller the study, the larger the benefit and
vice versa. In individual trials, relative risk benefits or hazard ratios reduced to
0.69 or 0.74. In the ANITA study, the number was 0.79 (Douillard 2005). In the largest study of all, the IALT study, it was 0.86 (Arriagada 2004). When
you look at them all together, the hazard ratio is about 0.9.
Dr Bria has also observed how many patients must be treated for one patient
to benefit, which is probably a good way to look at the issue. In his studies,
depending on which stage and which group of patients you look at, that
number is somewhere between 20 and 30 patients.
Select publications
|

